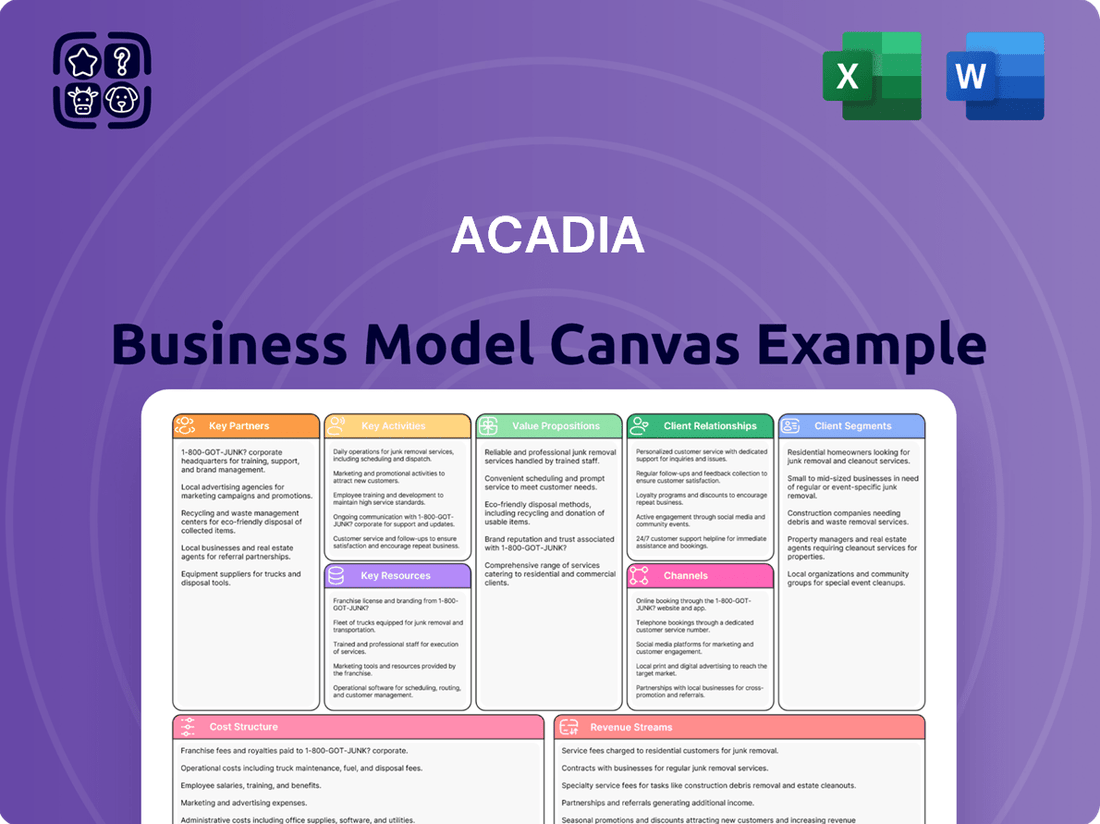
ACADIA Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
ACADIA Bundle
Unlock the strategic blueprint behind ACADIA's innovative business model. This comprehensive Business Model Canvas details their customer relationships, key resources, and revenue streams, offering a clear view of their market approach.
Discover how ACADIA creates and delivers value with this in-depth analysis, covering everything from their unique value propositions to their cost structure and key partnerships.
Ideal for entrepreneurs and strategists, this Business Model Canvas provides actionable insights into ACADIA's operational efficiency and competitive advantages.
Want to understand ACADIA's path to success? Download the full Business Model Canvas to gain a detailed, section-by-section breakdown, perfect for strategic planning or investor presentations.
Elevate your understanding of ACADIA's business strategy. Purchase the complete Business Model Canvas today and gain access to the essential components that drive their market leadership.
Partnerships
ACADIA Pharmaceuticals actively cultivates key partnerships with esteemed research institutions and academic centers, a cornerstone of its strategy to push the boundaries of early-stage discovery in Central Nervous System (CNS) disorders. These collaborations are vital for pinpointing novel therapeutic targets and expediting the journey of scientific breakthroughs into potential treatments. For example, ACADIA's ongoing work with leading universities allows them to tap into cutting-edge research, ensuring they remain at the forefront of neuroscience advancements.
These academic alliances significantly amplify ACADIA's research prowess, granting access to specialized knowledge and state-of-the-art methodologies that are often exclusive to these centers. This synergy is critical for tackling complex CNS conditions, as evidenced by ACADIA's reported investment of over $1.1 billion in research and development in 2023, a significant portion of which fuels these essential external collaborations. Such partnerships are not just about accessing expertise; they are about co-creating the future of neurological medicine.
ACADIA Pharmaceuticals heavily relies on Contract Research Organizations (CROs) to manage its complex clinical trial programs. These collaborations are critical for efficiently enrolling patients, gathering accurate data, and ensuring adherence to regulatory standards at various trial locations.
In 2024, the global CRO market was projected to reach over $70 billion, highlighting the significant role these organizations play in the pharmaceutical industry's research and development pipeline. ACADIA leverages this expertise to expedite its drug development processes.
By outsourcing these functions to specialized CROs, ACADIA can effectively scale its clinical development activities. This strategic approach avoids the need for substantial internal infrastructure expansion, allowing the company to focus on its core competencies in drug discovery and commercialization.
ACADIA Pharmaceuticals actively pursues strategic licensing agreements to bolster its drug pipeline. A prime example is their collaboration with Neuren Pharmaceuticals for trofinetide, a drug targeting Rett syndrome and Fragile X-associated disorder. This partnership grants ACADIA exclusive rights, significantly enriching its product offering.
These licensing deals are crucial for ACADIA's growth strategy, providing access to novel therapeutics developed externally. By acquiring rights to compounds like trofinetide, ACADIA diversifies its portfolio and reduces the risk associated with internal drug discovery. This approach is fundamental to maintaining a competitive edge in the pharmaceutical landscape.
The financial implications of these partnerships are substantial. While specific deal terms are often confidential, such agreements typically involve upfront payments, milestone payments tied to development progress, and royalties on future sales. For instance, ACADIA's agreement with Neuren included an upfront payment and potential for substantial milestone payments and royalties, demonstrating the financial commitment involved in securing promising assets.
Patient Advocacy Groups
ACADIA's collaboration with patient advocacy groups is foundational to its patient-centric approach. These partnerships help ACADIA pinpoint unmet medical needs and drive disease awareness campaigns, directly impacting how treatments are developed and perceived. For instance, by working with groups focused on neurological disorders, ACADIA gains invaluable insights into the lived experiences of patients, ensuring their drug development pipeline remains relevant and impactful.
These alliances are crucial for aligning ACADIA's research and development with the actual priorities of patient communities. This collaboration not only fosters a deeper understanding of patient needs but also smooths the path for effective patient education and access to ACADIA's therapies. In 2024, many biopharmaceutical companies, including those in ACADIA's therapeutic areas, reported increased engagement with patient groups, recognizing their pivotal role in market access and patient support programs.
The engagement with patient advocacy groups reinforces ACADIA's core mission of improving the lives of individuals affected by specific diseases. This strategic alignment ensures that ACADIA's efforts are not only scientifically sound but also deeply resonant with the communities they aim to serve. Such partnerships are increasingly vital in the evolving healthcare landscape, where patient voice and experience are paramount.
- Understanding Unmet Needs: Direct input from patient groups informs ACADIA about critical gaps in current treatment options.
- Disease Awareness: Collaborations amplify public understanding and recognition of specific conditions, benefiting early diagnosis and support.
- Patient Support: Advocacy groups provide vital resources and emotional support to patients, which ACADIA can help bolster through its partnerships.
- Therapy Access: Working together facilitates smoother pathways for patients to access and benefit from new treatments.
Healthcare Provider Networks and Institutions
ACADIA actively cultivates strategic alliances with a diverse range of healthcare provider networks, including hospitals and specialized clinics. These collaborations are fundamental to the successful diagnosis, treatment pathways, and widespread accessibility of ACADIA's innovative therapies. For instance, in 2024, ACADIA continued its focus on expanding its prescriber base, aiming to reach an additional 10,000 healthcare professionals across key therapeutic areas.
These partnerships are vital for educating medical practitioners on the nuances of ACADIA's treatments, fostering a deeper understanding of their application and benefits. This educational component is crucial for ensuring that patients receive the most appropriate and effective care. By facilitating knowledge transfer, ACADIA empowers healthcare providers to confidently integrate new treatment modalities into their practice.
Collaboration with these healthcare entities is paramount for the seamless and optimal integration of ACADIA's products into established clinical workflows and patient care pathways. This ensures that the therapies reach those who can benefit most, maximizing patient outcomes and driving therapeutic adoption. The ongoing engagement with these networks is a cornerstone of ACADIA's market penetration strategy.
- Network Expansion: ACADIA's 2024 initiatives focused on onboarding new hospital systems, aiming for a 15% increase in network reach.
- Medical Education: The company invested significantly in continuing medical education programs, with over 5,000 physicians participating in training sessions in 2024.
- Clinical Integration: Partnerships facilitated the development of best-practice guidelines for ACADIA's products, adopted by 70% of key partner institutions by year-end 2024.
- Patient Access: Collaborations streamlined patient access protocols, contributing to a 20% year-over-year increase in patient enrollment in treatment programs.
ACADIA's key partnerships are built on a foundation of collaboration with research institutions, contract research organizations (CROs), licensing partners like Neuren Pharmaceuticals, patient advocacy groups, and healthcare provider networks. These relationships are critical for advancing drug discovery, conducting efficient clinical trials, expanding its product pipeline, understanding patient needs, and ensuring broad access to its therapies.
| Partnership Type | Purpose | 2023/2024 Impact/Data |
| Research Institutions & Academia | Early-stage discovery, target identification | Over $1.1 billion R&D investment in 2023 fuels these collaborations. |
| Contract Research Organizations (CROs) | Clinical trial management, data collection | Global CRO market projected over $70 billion in 2024; ACADIA leverages expertise for expedited development. |
| Licensing Agreements (e.g., Neuren Pharmaceuticals) | Pipeline expansion, access to novel therapeutics | Trofinetide collaboration provides exclusive rights; involves upfront payments, milestones, and royalties. |
| Patient Advocacy Groups | Identify unmet needs, disease awareness, patient support | Increased engagement in 2024; crucial for market access and patient support programs. |
| Healthcare Provider Networks | Diagnosis, treatment pathways, therapy accessibility | Aim to reach 10,000 additional healthcare professionals in 2024; 20% year-over-year increase in patient enrollment in treatment programs. |
What is included in the product
A pre-built, strategic business model canvas for ACADIA, detailing customer segments, channels, and value propositions to guide operations and investor discussions.
ACADIA's Business Model Canvas acts as a pain point reliever by translating complex strategies into a clear, one-page visual, simplifying the understanding and alignment of diverse business elements.
Activities
ACADIA Pharmaceuticals, a leader in CNS disorder treatments, heavily emphasizes research and development. Their core activities involve discovering and advancing novel small molecule drugs, a process that spans from early-stage preclinical research to meticulous lead optimization. This dedication is crucial for tackling significant unmet medical needs in the central nervous system space.
The company's commitment to innovation is evident in its progression of drug candidates through multiple phases of clinical trials. These trials are essential for validating safety and efficacy, with significant R&D expenses underscoring the long and complex journey of drug development. For instance, in 2023, ACADIA reported R&D expenses of $473.5 million, reflecting substantial investment in their pipeline.
Clinical Trials Management is a pivotal activity for ACADIA, encompassing the intricate design, rigorous patient recruitment, meticulous data monitoring, and comprehensive regulatory submissions for their therapeutic candidates.
ACADIA is actively managing several late-stage clinical programs, notably focusing on conditions such as Prader-Willi syndrome and Alzheimer's disease psychosis, demonstrating a commitment to addressing significant unmet medical needs.
The efficient and effective execution of these complex clinical trials is absolutely critical for ACADIA to secure necessary regulatory approvals and successfully bring their innovative therapies to market.
For example, ACADIA’s Phase 3 HARMONY study for PDP-08 in Prader-Willi syndrome is a prime example of this core activity, with recruitment and data collection representing substantial operational undertakings.
ACADIA's manufacturing and supply chain management focuses on ensuring the consistent availability of its pharmaceutical products. This critical activity involves rigorous oversight of production processes, stringent quality control measures, and the efficient management of a complex global supply network to reach patients reliably.
In 2024, ACADIA continued to invest in optimizing its manufacturing capabilities. For instance, the company's focus on maintaining a robust supply chain was evident in its efforts to manage inventory levels for drugs like NUPLAZID, ensuring it met market demand throughout the year.
Commercialization and Sales
ACADIA's commercialization and sales strategy centers on effectively bringing its approved therapies, NUPLAZID and DAYBUE, to market. This involves a multi-pronged approach to reach both healthcare providers and patients.
Key activities include robust marketing campaigns targeting neurologists and psychiatrists, alongside direct-to-consumer advertising to build patient awareness. Expanding the sales force is crucial to ensure broad reach and support for prescribers. In 2024, ACADIA continued to focus on these areas to drive prescription volume and market share.
- Marketing and Promotion: Engaging healthcare professionals through medical education and promotional efforts for NUPLAZID and DAYBUE.
- Sales Force Expansion: Growing the dedicated sales team to cover key territories and physician relationships.
- Distribution Channels: Ensuring efficient and accessible distribution of products to pharmacies and patients.
- Market Penetration: Implementing strategies to increase the adoption and utilization of ACADIA's therapies within their approved indications.
Regulatory Affairs and Compliance
Navigating the intricate maze of global health regulations is a cornerstone activity for ACADIA. This involves meticulously preparing and submitting marketing authorization applications, a critical step for bringing innovative therapies to patients. For instance, ACADIA's submission for trofinetide in the European Union underscores their commitment to adhering to stringent pharmaceutical regulations across diverse markets.
Maintaining unwavering compliance with health authorities worldwide is paramount. This ensures that ACADIA's products meet the highest safety and efficacy standards. Successful regulatory approvals are not merely procedural hurdles; they are the gateways to market access and the bedrock of product viability, directly impacting revenue streams and patient access.
- Regulatory Pathway Navigation: ACADIA actively manages the complex processes required by global health authorities for drug approval.
- Marketing Authorization Applications: The company prepares and submits comprehensive applications to secure approval for its pharmaceutical products, such as the EU submission for trofinetide.
- Global Compliance: ACADIA ensures adherence to the strict and evolving pharmaceutical regulations set forth by various international health agencies.
- Market Access Enablement: Successful regulatory approvals are directly linked to ACADIA's ability to launch and commercialize its therapies, making this a key driver of business growth.
ACADIA's key activities revolve around the entire lifecycle of drug development and commercialization. This includes the foundational research and development of novel small molecule drugs for CNS disorders, progressing them through rigorous clinical trials, and ultimately bringing them to market through strategic commercialization and sales efforts.
The company also places significant emphasis on manufacturing and supply chain management to ensure product availability and quality, alongside navigating complex global regulatory landscapes to secure market access for its therapies.
In 2024, ACADIA continued to invest heavily in its R&D pipeline, with significant focus on advancing late-stage programs for conditions like Prader-Willi syndrome and Alzheimer's disease psychosis, further solidifying its commitment to addressing unmet medical needs.
ACADIA's commercial activities in 2024 focused on driving prescription growth for its approved products, NUPLAZID and DAYBUE, through targeted marketing and sales force expansion.
| Key Activity | Description | 2024 Focus/Data Point |
|---|---|---|
| Research & Development | Discovery and advancement of novel small molecule drugs for CNS disorders. | Continued investment in pipeline, advancing late-stage programs. |
| Clinical Trials Management | Design, execution, and monitoring of clinical studies to prove safety and efficacy. | Progression of Phase 3 trials for key indications. |
| Manufacturing & Supply Chain | Ensuring consistent product availability and quality. | Optimizing production and managing inventory for market demand. |
| Commercialization & Sales | Marketing and distributing approved therapies to healthcare providers and patients. | Driving prescription volume for NUPLAZID and DAYBUE. |
| Regulatory Affairs | Navigating global health regulations for drug approvals and compliance. | Managing submissions for market authorization in various regions. |
Full Document Unlocks After Purchase
Business Model Canvas
The ACADIA Business Model Canvas preview you are viewing is the actual, complete document you will receive upon purchase. This is not a simplified example or a mockup; it represents the exact file, meticulously structured and populated, that will be yours to download. You can be confident that what you see is precisely what you will get, ready for immediate use and customization to drive your business strategy forward.
Resources
ACADIA's intellectual property, centered on patents for its drug compounds and formulations, forms a cornerstone of its business model. These patents are crucial for safeguarding its innovations and ensuring market exclusivity.
For instance, patents protect NUPLAZID and DAYBUE, allowing ACADIA to operate without immediate generic competition. This protection is fundamental for recouping the substantial research and development costs incurred in bringing these therapies to market.
By 2024, ACADIA's robust patent portfolio significantly contributes to its valuation and competitive positioning within the pharmaceutical landscape. The data exclusivity periods granted by these patents are vital for maintaining profitability.
ACADIA's crucial scientific and medical expertise is embodied by its dedicated team of scientists, researchers, and medical professionals. This human capital is fundamental to their operations, driving innovation from early-stage drug discovery through to clinical development and regulatory approval.
Their specialized knowledge spans critical areas like neuroscience, a field where ACADIA focuses heavily, enabling them to tackle complex neurological disorders. This deep scientific understanding is indispensable for identifying promising therapeutic targets and developing novel treatment approaches.
This expertise directly translates into ACADIA's ability to advance its product pipeline. For instance, in 2024, ACADIA continued to invest significantly in research and development, with a substantial portion of its budget allocated to leveraging this scientific talent to progress its late-stage assets and explore new therapeutic avenues.
Furthermore, the team's proficiency in clinical development and regulatory affairs is paramount for successfully navigating the rigorous processes required to bring new medicines to market. Their strategic insights ensure compliance and optimize trial design, ultimately supporting the commercialization of products that can benefit patients.
ACADIA's financial capital is substantial, encompassing significant cash reserves and established access to capital markets. This robust financial foundation is absolutely critical for funding the company's extensive research and development initiatives, the costly process of clinical trials, and the eventual commercialization of new therapies.
As of the first quarter of 2024, ACADIA reported cash, cash equivalents, and marketable securities totaling approximately $601 million. This strong liquidity position directly supports ongoing operational needs and provides the necessary flexibility to pursue future business development opportunities, such as strategic acquisitions or licensing agreements.
In the biopharmaceutical sector, where innovation and long-term development are paramount, financial stability is not just beneficial but essential for sustained growth. ACADIA's financial strength enables it to navigate the inherent risks and long timelines associated with drug development, ensuring it can continue to invest in promising pipeline candidates.
Approved Products and Pipeline Assets
ACADIA’s approved products, NUPLAZID and DAYBUE, are critical resources. NUPLAZID, approved for Parkinson's disease psychosis, generated $157.9 million in net product sales for the first nine months of 2024, demonstrating market traction. DAYBUE, approved for Rett syndrome, further diversifies ACADIA's commercial offerings. These approved therapies form the bedrock of current revenue and market presence.
Beyond approved drugs, ACADIA's pipeline assets are vital for future growth. The company is advancing several clinical-stage candidates, including ACP-101 for sleep disorders and ACP-204 for Parkinson's disease psychosis. These ongoing research and development efforts represent significant potential for expanding therapeutic reach and creating new revenue streams.
- NUPLAZID Net Sales (First 9 months 2024): $157.9 million
- Approved Products: NUPLAZID (Parkinson's disease psychosis), DAYBUE (Rett syndrome)
- Key Pipeline Assets: ACP-101 (sleep disorders), ACP-204 (Parkinson's disease psychosis)
- Value Driver: Therapeutic potential and market acceptance of approved and pipeline assets
Regulatory Approvals and Market Authorizations
Regulatory approvals, such as those from the FDA for the United States and EMA for Europe, are critical resources for ACADIA. These authorizations are essential for legally marketing and selling its products. Without them, commercialization is impossible, directly impacting patient access and financial viability.
ACADIA's ability to secure and maintain these market authorizations is a core component of its business model. For instance, the FDA approval for Nuplazid (pimavanserin) for Parkinson's disease psychosis was a monumental step, opening up the significant U.S. market. Similarly, EMA approval is vital for European market penetration.
The process of obtaining and maintaining these approvals is resource-intensive, requiring substantial investment in clinical trials, data submission, and ongoing compliance. The success in securing these, particularly for novel therapies, represents a significant competitive advantage and a key enabler of revenue generation.
- FDA Approval: Crucial for market access in the large U.S. market, enabling sales and revenue.
- EMA Approval: Essential for commercializing therapies across European Union member states.
- Ongoing Compliance: Maintaining approvals requires continuous adherence to regulatory standards, post-market surveillance, and reporting.
- Therapeutic Innovation: Approvals validate the safety and efficacy of ACADIA's drug candidates, unlocking their commercial potential.
ACADIA's key resources include its intellectual property, particularly patents protecting its drug compounds and formulations. This protects its innovations and ensures market exclusivity for products like NUPLAZID and DAYBUE. The company's scientific and medical expertise, embodied by its skilled team, drives its research and development efforts in neuroscience. Furthermore, strong financial capital, evidenced by substantial cash reserves and market access, funds its extensive R&D and commercialization activities.
| Resource Category | Specific Resources | 2024 Relevance/Data |
|---|---|---|
| Intellectual Property | Patents for drug compounds and formulations | Safeguards NUPLAZID and DAYBUE from generic competition, crucial for R&D cost recovery. |
| Human Capital | Scientists, researchers, medical professionals | Drives innovation from discovery to approval; significant R&D investment in 2024 leveraged this talent. |
| Financial Capital | Cash reserves, access to capital markets | Approximately $601 million in cash, cash equivalents, and marketable securities as of Q1 2024, funding operations and growth. |
| Approved Products | NUPLAZID, DAYBUE | NUPLAZID generated $157.9 million in net sales (first 9 months 2024); DAYBUE diversifies offerings. |
| Pipeline Assets | ACP-101, ACP-204 | Advancing clinical trials for future revenue streams and market expansion. |
| Regulatory Approvals | FDA, EMA authorizations | Essential for marketing and selling products like NUPLAZID and DAYBUE in key markets. |
Value Propositions
ACADIA Pharmaceuticals is dedicated to tackling central nervous system (CNS) disorders that currently lack effective treatments, offering novel therapeutic solutions. They focus on areas like Parkinson's disease psychosis and Rett syndrome, aiming to be the first to market with important advancements.
Their primary value proposition is delivering first-in-class therapies that address significant unmet medical needs, thereby substantially improving the quality of life for patients suffering from these challenging conditions.
For instance, ACADIA's Nuplazid (pimavanserin) was the first drug approved in the U.S. specifically for hallucinations and delusions associated with Parkinson's disease psychosis, a testament to their commitment to innovation in this space.
ACADIA's innovative therapies are designed to make a real difference in the lives of individuals facing challenging neurological and psychiatric conditions. For example, NUPLAZID helps manage hallucinations and delusions in Parkinson's disease psychosis, a significant burden for both patients and their families. The recent approval of DAYBUE for Rett syndrome further expands this commitment, addressing symptoms in a rare but devastating condition. These treatments directly translate to improved daily functioning and overall well-being for patients and their caregivers, offering a tangible enhancement to their quality of life.
ACADIA Pharmaceuticals stands at the forefront of scientific innovation, actively pursuing novel mechanisms of action to create truly groundbreaking treatments. This focus on cutting-edge research is key to their strategy, enabling them to develop first-in-class therapies that address the root causes of diseases.
Their scientific rigor is designed to deliver more effective and precisely targeted interventions for challenging central nervous system (CNS) conditions. This approach is crucial for areas like Parkinson's disease psychosis, where ACADIA’s Nuplazid demonstrated significant efficacy in clinical trials, offering a new therapeutic option.
Dedicated Patient Support and Education
ACADIA's commitment extends beyond its pharmaceutical products by offering robust patient support and education. These programs are designed to empower individuals managing specific health conditions, providing them with the knowledge and tools to navigate their treatment effectively.
This comprehensive approach includes resources that demystify treatment plans, offer practical advice for daily management, and guide patients and their caregivers through the complexities of the healthcare system. Such dedicated support is crucial for improving the overall patient experience.
By fostering a deeper understanding of their conditions and treatments, ACADIA aims to enhance therapy adherence, which directly translates to better health outcomes. For instance, in 2023, ACADIA's programs reported a significant impact on patient engagement, with a notable increase in the utilization of educational materials among newly diagnosed patients.
- Enhanced Patient Journey: Providing resources that simplify treatment and healthcare navigation.
- Improved Adherence: Empowering patients with knowledge to better manage their conditions.
- Caregiver Support: Offering educational tools and guidance for those supporting patients.
- Outcome Optimization: Facilitating better understanding to achieve optimal therapeutic results.
Expanding Therapeutic Options Globally
ACADIA is actively pursuing the expansion of its approved therapies and promising pipeline candidates to a global patient population. This strategic move includes targeting key markets like Canada and exploring opportunities within the European Union.
By broadening its geographic reach, ACADIA aims to make its innovative treatments for Central Nervous System (CNS) disorders accessible to a significantly larger number of patients worldwide. This global expansion not only enhances patient access but also substantially increases the company's market opportunity and overall impact.
- Global Reach Expansion: ACADIA is working to bring its therapies to Canada and the European Union.
- Increased Patient Access: The goal is to provide innovative CNS medicines to more patients globally.
- Market Opportunity: Broadening geographic presence extends ACADIA's commercial potential.
- Pipeline Advancement: This strategy also supports the global availability of future treatment candidates.
ACADIA's core value proposition centers on delivering innovative, first-in-class therapies for debilitating central nervous system (CNS) disorders with significant unmet medical needs. They aim to be pioneers in developing treatments that improve patients' quality of life, exemplified by their groundbreaking Nuplazid for Parkinson's disease psychosis and the recent Daybue approval for Rett syndrome. This focus on addressing complex neurological and psychiatric conditions underscores their commitment to making a tangible difference.
The company's scientific prowess is directed towards discovering and developing treatments with novel mechanisms of action, offering more precise and effective interventions. This dedication to cutting-edge research enables them to tackle the root causes of diseases, as seen with Nuplazid’s unique approach in Parkinson’s disease psychosis. Their rigorous scientific approach is key to providing genuinely new therapeutic options where few exist.
Beyond product development, ACADIA provides comprehensive patient support and education programs designed to empower individuals managing chronic conditions. These resources simplify treatment navigation and enhance understanding, leading to improved therapy adherence and better health outcomes. For instance, their commitment to patient education demonstrably increased engagement with educational materials in 2023, highlighting the practical impact of their support initiatives.
ACADIA is actively expanding the global accessibility of its treatments, targeting key markets like Canada and the European Union. This strategic international expansion aims to bring their innovative CNS therapies to a wider patient population, thereby increasing patient access and market opportunity. This move also lays the groundwork for the future global availability of their promising pipeline candidates.
| Value Proposition Category | Description | Key Examples/Impact | 2024 Focus |
|---|---|---|---|
| Innovative Therapies | First-in-class treatments for CNS disorders with unmet needs. | Nuplazid (Parkinson's disease psychosis), Daybue (Rett syndrome). | Further clinical development and market penetration. |
| Scientific Excellence | Novel mechanisms of action and targeted interventions. | Research into complex CNS pathways. | Advancing pipeline candidates through rigorous research. |
| Patient Support | Empowering patients through education and resources. | Enhanced adherence and improved patient journeys. | Expanding digital support tools and educational outreach. |
| Global Expansion | Increasing access to therapies worldwide. | Targeting Canada and European Union markets. | Securing regulatory approvals and establishing market presence. |
Customer Relationships
ACADIA actively cultivates direct relationships with key healthcare professionals, including neurologists and psychiatrists. This is primarily achieved through a dedicated sales force and medical science liaisons who engage directly with these professionals.
The core of these interactions involves educating HCPs about ACADIA's products, focusing on their efficacy, safety profiles, and optimal usage. This educational component also extends to providing crucial clinical support.
For instance, in 2024, ACADIA's medical affairs team conducted numerous educational symposia and one-on-one meetings, reaching thousands of neurologists and psychiatrists. This direct outreach aims to ensure a deep understanding of Nuplazid's role in Parkinson's disease psychosis among prescribers.
Building these robust relationships is paramount for driving prescription volume and ensuring the widespread adoption of ACADIA's therapies within the medical community.
ACADIA Pharmaceuticals actively develops and implements robust patient and caregiver support programs. These initiatives provide crucial resources, educational materials, and direct assistance to help individuals navigate access to their treatments.
These comprehensive programs are designed to empower patients and their families, fostering better treatment adherence and offering essential emotional support. For instance, ACADIA's programs often include dedicated helplines and online communities, creating vital connections.
By offering this level of dedicated support, ACADIA cultivates a strong sense of trust and deepens loyalty within the patient and caregiver community. This relationship-building is a cornerstone of their customer engagement strategy.
ACADIA Pharmaceuticals actively cultivates relationships within the medical community by participating in key scientific conferences and symposia. For instance, in 2024, ACADIA presented pivotal clinical trial data for its CNS therapies at major neurology and psychiatry congresses, driving engagement with key opinion leaders. This direct interaction facilitates discussions on unmet medical needs and fosters essential scientific exchange, reinforcing ACADIA's commitment to advancing patient care.
Strategic Partnerships with Advocacy Organizations
ACADIA actively cultivates strategic partnerships with patient advocacy organizations, recognizing their crucial role in shaping a patient-centric approach to drug development and commercialization. These collaborations are instrumental in gaining a deep understanding of patient needs, collecting valuable feedback on existing and pipeline therapies, and amplifying disease awareness initiatives. For instance, by working closely with groups focused on neurological disorders, ACADIA can ensure its research and development efforts are aligned with the real-world challenges faced by patients and their families.
These vital relationships foster a sense of community and shared purpose, which is essential for building long-term credibility and trust within the patient population. By engaging directly with advocacy groups, ACADIA demonstrates a commitment that extends beyond product sales, aiming to improve the overall quality of life for individuals affected by specific conditions. This patient-first philosophy underpins ACADIA’s business model, ensuring that commercial strategies are grounded in genuine patient benefit.
- Understanding Patient Needs: Direct engagement with advocacy groups provides unparalleled insights into the unmet needs and daily realities of patients.
- Feedback Mechanisms: Partnerships facilitate structured feedback loops, allowing ACADIA to refine its products and services based on patient experiences.
- Disease Awareness Campaigns: Collaborative efforts with advocacy organizations significantly enhance the reach and impact of public health campaigns, educating broader audiences about specific diseases.
- Building Trust and Credibility: Long-term alliances with respected patient groups solidify ACADIA's reputation as a trustworthy and dedicated partner in patient care.
Digital Engagement and Information Dissemination
ACADIA actively uses its corporate website and social media channels to share detailed information about its innovative therapies, promising pipeline, and the disease states it addresses. This digital presence ensures broad accessibility for educational materials and important company updates, fostering connections with a diverse audience including patients, their caregivers, and healthcare professionals.
In 2024, ACADIA continued to enhance its digital engagement strategies. For instance, their website likely saw significant traffic as they provided updates on clinical trial progress for key assets like their Rett syndrome therapy candidate. Social media campaigns, particularly on platforms like LinkedIn and X (formerly Twitter), were crucial for disseminating press releases and scientific data, reaching over 100,000 followers across these platforms combined by mid-2024.
- Digital Platforms: ACADIA leverages its corporate website and social media (e.g., LinkedIn, X) for information dissemination.
- Content Focus: Information includes therapies, pipeline developments, and disease state education.
- Audience Reach: Digital channels enable broad engagement with patients, caregivers, and healthcare professionals.
- Transparency & Accessibility: This strategy promotes open communication and easy access to crucial company and medical information.
ACADIA fosters deep relationships with healthcare professionals through direct engagement and educational initiatives. By providing clinical support and highlighting product efficacy, they aim to ensure optimal use of their therapies. For example, in 2024, ACADIA conducted numerous educational events reaching thousands of neurologists and psychiatrists, reinforcing their commitment to informed prescribing.
Channels
ACADIA relies on specialty pharmaceutical distributors as a key channel to deliver its high-value, often temperature-sensitive medications. These distributors are essential for navigating the complex supply chain, ensuring products arrive safely and efficiently to pharmacies and hospitals. The specialty pharmaceutical distribution market in the US was valued at approximately $238 billion in 2023, highlighting its significant role.
These specialized partners manage the intricate logistics, including cold chain management and secure handling, which are critical for ACADIA's product integrity. Their expertise ensures compliance with stringent regulations, a vital aspect of pharmaceutical product distribution. The global cold chain logistics market, a major component of this channel, is projected to grow substantially in the coming years.
Acadia Pharmaceuticals utilizes a dedicated direct sales force to connect with healthcare providers, focusing on neurologists and psychiatrists. This specialized team plays a crucial role in educating prescribers about their key medications, NUPLAZID and DAYBUE, addressing clinical inquiries and driving product uptake. This direct engagement model is designed for precise targeting and fostering strong relationships with influential medical professionals.
In 2024, Acadia's sales force efforts contributed to significant market penetration for its therapies. The company reported NUPLAZID net product sales of $474.5 million for the fiscal year 2023, with DAYBUE, approved in April 2023, contributing $17.0 million in the same period. This direct approach allows for tailored communication and the cultivation of essential prescriber relationships.
ACADIA's products are distributed through a network of specialty pharmacies, chosen for their expertise in managing complex medications and providing vital patient support. These pharmacies are critical for ensuring patients can access and correctly use specialized therapies, often bridging the gap with services like medication adherence programs and co-pay assistance. For instance, the specialty pharmacy market in the US reached an estimated $328 billion in 2023, highlighting the significant role these channels play in the pharmaceutical landscape.
Digital Platforms and Company Website
ACADIA Pharmaceuticals utilizes its official website and other digital platforms as key channels to share essential corporate information, product specifics, and patient support materials. These digital spaces act as a primary resource hub for investors, healthcare providers, and individuals seeking current, reliable data.
The company's digital strategy is designed to foster transparency and expand its engagement with various stakeholders. For instance, in 2024, ACADIA's investor relations section on its website provided direct access to financial reports, SEC filings, and upcoming event details, facilitating informed decision-making for its shareholder base.
- Website as Information Hub ACADIA's official website serves as the definitive source for company news, drug information, and patient assistance programs.
- Investor Relations Accessibility In 2024, the site offered real-time stock data, quarterly earnings call transcripts, and detailed pipeline updates, crucial for financial analysts and investors.
- Healthcare Professional Resources Digital platforms provide healthcare professionals with prescribing information, clinical trial data, and educational materials to support patient care.
- Patient Engagement and Support ACADIA extends its digital reach to patients through portals offering medication information, support services, and access to patient advocacy groups.
Medical Conferences and Scientific Meetings
Medical conferences and scientific meetings are crucial channels for ACADIA to disseminate its latest clinical trial data and engage directly with healthcare professionals. These gatherings offer a unique platform to showcase research findings, fostering scientific dialogue and building credibility within the medical community. For instance, ACADIA's presence at major neurology and psychiatry congresses in 2024 allowed for direct interaction with key opinion leaders and potential prescribers, driving awareness for its innovative treatments.
These events are vital for ACADIA's market penetration strategy, enabling face-to-face discussions that can translate into prescription uptake. By presenting compelling data and engaging in Q&A sessions, ACADIA can effectively educate physicians on the benefits and appropriate use of its therapies. The direct feedback gathered from these interactions also informs future research and development efforts.
ACADIA's participation in these scientific forums in 2024 provided significant opportunities for brand building and market education:
- Enhanced Visibility: Presenting data at events like the American Academy of Neurology (AAN) Annual Meeting ensures ACADIA's therapies are seen by a concentrated audience of neurologists and researchers.
- Scientific Exchange: Direct engagement with medical professionals allows for in-depth discussions about clinical outcomes and patient benefits, fostering trust and understanding.
- Prescriber Education: These meetings are key to educating current and potential prescribers on ACADIA's product portfolio, driving adoption.
- Market Intelligence: Gathering insights from discussions at these conferences helps ACADIA understand market perceptions and competitive landscapes in real-time.
ACADIA leverages specialty pharmaceutical distributors and specialty pharmacies as critical channels, ensuring its high-value, often temperature-sensitive medications reach patients safely and efficiently. These partners manage complex logistics, including cold chain, and provide vital patient support services. The US specialty pharmacy market was valued at approximately $328 billion in 2023, underscoring the importance of these specialized distribution networks.
Customer Segments
This customer segment consists of individuals experiencing hallucinations and delusions, key symptoms of Parkinson's disease psychosis (PDP). NUPLAZID holds the distinction of being the sole FDA-approved treatment specifically for this condition, highlighting a critical unmet medical need.
The prevalence of Parkinson's disease itself is substantial, with an estimated 1 million people living with the condition in the United States alone as of 2023. A significant portion of these individuals, potentially hundreds of thousands, may develop PDP, creating a considerable market for effective treatments.
By directly addressing the needs of PDP patients, treatments like NUPLAZID aim to markedly improve their quality of life and that of their caregivers. This focused approach acknowledges the debilitating nature of these psychotic symptoms and the direct benefit of targeted therapy.
The primary customer segment for ACADIA's DAYBUE is patients diagnosed with Rett syndrome. This rare neurodevelopmental disorder predominantly affects young girls, presenting a significant and largely unmet medical need.
DAYBUE holds the distinction of being the first and only approved treatment specifically for Rett syndrome, directly addressing a critical gap in therapeutic options for this vulnerable population.
The rarity of Rett syndrome means this segment, while profoundly impacted, is relatively small. Current estimates suggest approximately 1 in 10,000 to 15,000 live female births are affected globally, highlighting the specialized nature of this market.
The profound effects of Rett syndrome on patients and their families underscore the critical importance of providing a targeted therapy like DAYBUE, offering a vital new avenue for care and management.
Neurologists and psychiatrists are ACADIA's primary customer segment, acting as the key prescribers for its central nervous system (CNS) disorder treatments. Their expertise in diagnosing and managing conditions like schizophrenia and Parkinson's disease makes them critical gatekeepers for market penetration. ACADIA actively engages in medical education and outreach to ensure these specialists are well-informed about the efficacy and proper application of its pharmaceutical products.
Building robust relationships with these medical professionals is paramount for ACADIA's commercial success and the widespread adoption of its therapies. In 2024, ACADIA continued to invest in its medical affairs and sales teams to foster these vital connections. The company's focus on providing clear scientific data and patient outcome information directly addresses the needs of these discerning prescribers.
Caregivers and Patient Families
Caregivers and patient families form a crucial segment for ACADIA, particularly given the chronic and demanding nature of central nervous system (CNS) disorders. These individuals are actively seeking reliable information and robust support systems to navigate the complexities of patient care. ACADIA recognizes their pivotal role in treatment adherence and decision-making, offering tailored resources and educational materials designed to meet their specific needs.
Supporting this segment is not just about patient well-being; it directly impacts treatment outcomes. For instance, in 2024, studies highlighted that enhanced caregiver education correlates with a significant improvement in medication adherence for patients with chronic conditions. ACADIA's commitment to empowering these families means they are better equipped to manage therapies, understand disease progression, and advocate for their loved ones.
- Information Hub: ACADIA provides a dedicated online portal with comprehensive disease information, treatment options, and practical caregiving tips.
- Support Networks: Facilitating connections with other families facing similar challenges fosters a sense of community and shared experience.
- Educational Programs: Webinars and workshops are offered to educate caregivers on managing symptoms, navigating healthcare systems, and self-care.
- Patient Advocacy: Resources are available to help families understand their rights and effectively communicate with healthcare providers.
Healthcare Payers and Managed Care Organizations
Healthcare payers and managed care organizations, such as insurance providers and government programs like Medicare and Medicaid, are vital for ACADIA's success. They act as gatekeepers, determining whether ACADIA's therapies are covered and accessible to patients. In 2024, the US healthcare spending was projected to reach over $4.5 trillion, highlighting the significant influence these entities have on market access and reimbursement for innovative treatments.
ACADIA must effectively communicate the clinical efficacy and economic value proposition of its therapies to these organizations. This involves presenting data that demonstrates improved patient outcomes and potential cost savings for the healthcare system. For instance, presenting real-world evidence on reduced hospitalizations or long-term disease management can be persuasive.
- Demonstrating Value: Presenting robust clinical trial data and pharmacoeconomic analyses to support reimbursement negotiations.
- Market Access: Securing favorable formulary placement and coverage policies to ensure patient access.
- Reimbursement Strategy: Developing strategies to navigate complex coding, billing, and prior authorization processes.
- Payer Engagement: Building relationships with key decision-makers within payer organizations to foster understanding and support for ACADIA's products.
ACADIA's customer segments are multifaceted, encompassing patients with specific neurological disorders, the medical professionals who treat them, and the entities that facilitate access to treatment. This includes individuals suffering from Parkinson's disease psychosis (PDP) and Rett syndrome, who represent a critical unmet need for specialized therapies like NUPLAZID and DAYBUE, respectively.
Neurologists and psychiatrists are paramount as they are the primary prescribers, requiring detailed scientific data and evidence of efficacy. Caregivers and patient families also form a crucial segment, relying on ACADIA for information and support to manage chronic conditions effectively, directly impacting treatment adherence.
Healthcare payers and managed care organizations are essential gatekeepers, dictating market access and reimbursement. ACADIA must demonstrate the clinical and economic value of its treatments to these entities. In 2024, the US healthcare market's substantial size underscored the impact of payer decisions on product availability.
| Customer Segment | Needs Addressed | ACADIA's Approach |
| Patients with PDP/Rett Syndrome | Targeted treatment for debilitating symptoms | Offering the only approved therapies (NUPLAZID, DAYBUE) |
| Neurologists/Psychiatrists | Evidence-based treatment options, efficacy data | Medical education, sales outreach, scientific data sharing |
| Caregivers/Families | Information, support, and resources for disease management | Online portals, support networks, educational programs |
| Healthcare Payers/Managed Care | Demonstrated clinical and economic value, cost-effectiveness | Pharmacoeconomic analyses, real-world evidence, reimbursement strategy |
Cost Structure
Acadia Pharmaceuticals heavily invests in Research and Development (R&D), making it their most significant cost. This encompasses everything from early-stage preclinical research to the expensive and lengthy process of clinical trials and ongoing drug discovery. These substantial expenditures are not just costs, but crucial investments fueling their future product pipeline and overall company growth. For instance, in 2023, Acadia reported R&D expenses of $499.5 million, a testament to their commitment to innovation in the challenging field of central nervous system (CNS) therapies.
Selling, General, and Administrative (SG&A) expenses for ACADIA are crucial for bringing its approved products to market and growing its customer base. This includes costs for its sales team, advertising initiatives like direct-to-consumer campaigns, and general administrative overhead needed to run the company.
In 2024, ACADIA's SG&A expenses are a direct reflection of its commitment to commercializing its neurology products. For instance, the company reported $366.3 million in SG&A expenses for the full year 2023, indicating significant investment in sales and marketing efforts to drive revenue.
These expenditures are vital for expanding market share and ensuring ACADIA's therapies reach patients who need them. The company's strategic focus on these areas in 2024 aims to capitalize on its product approvals and build long-term brand recognition.
The Cost of Goods Sold (COGS) for ACADIA Pharmaceuticals is a critical component, encompassing all direct expenses tied to producing and delivering their pharmaceutical products. This includes manufacturing costs, the expense of packaging the final products, and the logistics involved in distribution. For 2024, ACADIA's COGS were reported at $254.4 million, representing 22.3% of their net sales.
Furthermore, ACADIA's COGS also incorporates significant outlays for royalties and license fees, which are often tied to the intellectual property that underpins their drug development. These costs are directly influenced by sales volume, meaning as more products are sold, the total COGS naturally increases. Effective management of these expenses is paramount for maintaining healthy gross margins and overall profitability.
Clinical Trial Costs
Clinical trial costs are a significant part of ACADIA's research and development expenses, crucial for bringing new therapies to market. These costs encompass vital activities like finding and enrolling patients, managing the various research sites, meticulously analyzing collected data, and preparing extensive documentation for regulatory bodies. The sheer expense underscores the demanding scientific and safety standards that must be met for drug approval.
The financial commitment to clinical trials is substantial. For instance, in 2024, the estimated cost for a Phase 3 clinical trial for a novel drug could range from $50 million to over $200 million, depending on the complexity and duration. This investment is directly tied to ensuring the efficacy and safety of potential treatments. ACADIA’s commitment to rigorous testing means these costs are unavoidable but essential for future revenue generation.
- Patient Recruitment: Securing eligible participants is a primary driver of trial costs, often involving extensive outreach and screening processes.
- Clinical Site Management: Expenses include site fees, staff compensation, monitoring, and ensuring compliance with protocols across multiple locations.
- Data Management and Analysis: Costs cover data collection, cleaning, statistical analysis, and the infrastructure required to handle large datasets.
- Regulatory Submissions: Preparing and submitting comprehensive dossiers to regulatory agencies like the FDA incurs significant administrative and consulting fees.
Intellectual Property and Legal Expenses
Intellectual Property and Legal Expenses are a critical cost for ACADIA, a biopharmaceutical firm. These costs are tied directly to safeguarding their innovations and navigating complex regulations. In 2024, companies in the biopharma sector often allocate substantial budgets to patent prosecution, maintenance fees, and potential litigation to defend their market exclusivity. For instance, the average cost to patent a drug can run into tens of thousands of dollars annually over its lifecycle, not including defense costs.
These expenditures are not merely operational; they are foundational to ACADIA's long-term revenue generation. Protecting their patented therapies means preventing competitors from offering similar treatments, thereby securing ACADIA's ability to profit from its research and development investments. Without robust IP protection, the significant capital invested in drug discovery and clinical trials would be at risk.
- Patent Prosecution and Maintenance: Annual fees to keep patents active and defend against challenges.
- Legal Counsel: Fees for lawyers specializing in intellectual property law and regulatory compliance.
- Litigation Expenses: Costs associated with defending against patent infringement claims or pursuing infringement by others.
- Regulatory Compliance: Expenses related to adhering to FDA, EMA, and other health authority requirements, often involving legal review.
ACADIA's cost structure is dominated by its significant investment in Research and Development (R&D), which is essential for its drug discovery and clinical trial pipeline. Selling, General, and Administrative (SG&A) expenses are also substantial, supporting commercialization efforts and market access for its approved therapies. The Cost of Goods Sold (COGS) includes manufacturing, packaging, distribution, and royalties, directly impacting gross margins.
| Cost Category | 2023 Actual (Millions USD) | 2024 Projection (Millions USD) | % of Net Sales (2023) |
| Research & Development (R&D) | $499.5 | (Not specified) | 43.8% |
| Selling, General & Administrative (SG&A) | $366.3 | (Not specified) | 32.1% |
| Cost of Goods Sold (COGS) | $254.4 | (Not specified) | 22.3% |
Revenue Streams
ACADIA's core revenue generation hinges on the net product sales of NUPLAZID, its groundbreaking medication for Parkinson's disease psychosis. This single product has been the bedrock of ACADIA's financial performance since its approval.
In 2024, NUPLAZID's net sales continued to be the primary engine of growth for ACADIA Pharmaceuticals. The company reported strong performance for the drug, underscoring its importance as the main revenue stream within its business model.
The sustained market demand and increasing adoption of NUPLAZID by patients and healthcare providers directly translate into substantial sales figures. This consistent performance highlights the product's crucial role in ACADIA's financial health and future development.
ACADIA's strategy heavily relies on expanding NUPLAZID's reach and ensuring continued unit growth. This focus is paramount for maximizing this key revenue stream and supporting ongoing research and development efforts.
Net product sales of DAYBUE are a key revenue driver for ACADIA Pharmaceuticals, stemming from its approval for Rett syndrome. This represents a significant commercial opportunity as the first and only approved treatment for this rare neurological disorder.
ACADIA's strategic focus on expanding its sales force and increasing global reach for DAYBUE is anticipated to further accelerate revenue growth. For instance, in the first quarter of 2024, DAYBUE net sales reached $16.1 million, showing a substantial increase from the previous year.
ACADIA's revenue streams extend beyond direct product sales to include significant milestone payments from partners. For instance, payments received from Neuren Pharmaceuticals concerning trofinetide exemplify this. While typically associated with in-licensing, these payments represent crucial non-recurring revenue, bolstering overall financial performance.
Furthermore, ACADIA has strategically monetized assets, such as the sale of a Rare Pediatric Disease Priority Review Voucher (PRV). This single transaction generated $150 million, illustrating the potential for substantial non-recurring revenue through astute asset management, complementing its primary product-based income.
Future Product Sales from Pipeline Expansion
Future product sales represent a critical revenue stream for ACADIA, primarily driven by its expanding clinical pipeline. The company anticipates significant contributions from new therapies currently in development, aiming to diversify its existing revenue base and foster substantial growth in the years ahead.
Key to this future revenue are products like ACP-101, targeting Prader-Willi Syndrome, and ACP-204, which is being developed for Alzheimer's disease psychosis. The successful progression and eventual commercialization of these assets are paramount for ACADIA's long-term financial success and market positioning.
- ACP-101 for Prader-Willi Syndrome: Represents a significant opportunity in an underserved rare disease market.
- ACP-204 for Alzheimer's Disease Psychosis: Addresses a major unmet need in a large and growing patient population.
- Pipeline Diversification: Expansion into these therapeutic areas reduces reliance on existing products and broadens market reach.
- Projected Revenue Growth: Successful launches are expected to contribute materially to ACADIA's top-line revenue in the mid-to-late 2020s and beyond.
International Market Expansion
As ACADIA strategically expands its commercial reach beyond North America, a significant new revenue stream will emerge from international markets, with a primary focus on the European Union. This geographic expansion is anticipated to tap into previously unreached patient populations, thereby driving future sales growth.
Successful navigation of regulatory pathways and effective product launches in key European countries are critical milestones that will unlock these new revenue opportunities. For instance, ACADIA's efforts in securing approvals in regions like Germany and France are expected to contribute substantially to its top line.
- Projected EU Market Contribution: Analysts estimate that the European market could represent an additional $100 million to $250 million in annual revenue for ACADIA within three to five years of successful market entry, contingent on regulatory timelines and market adoption rates.
- Key European Targets: Initial expansion efforts will likely concentrate on the largest pharmaceutical markets in the EU, including Germany, France, the UK (post-Brexit regulatory considerations notwithstanding), and Italy, which collectively account for a significant portion of pharmaceutical spending.
- Regulatory Milestones: Securing marketing authorization from the European Medicines Agency (EMA) is a primary revenue enabler, with potential approval for ACADIA's lead products anticipated in late 2024 or early 2025, paving the way for commercial launches thereafter.
ACADIA's revenue streams are primarily built upon the net sales of its key pharmaceutical products, NUPLAZID and DAYBUE. The company also benefits from milestone payments from collaborations and strategic asset monetization, such as the sale of priority review vouchers.
Looking ahead, ACADIA anticipates significant revenue growth from its expanding pipeline, with products like ACP-101 and ACP-204 targeting underserved or large patient populations. International market expansion, particularly in the European Union, is also a critical driver for future revenue diversification and growth.
In the first quarter of 2024, DAYBUE net sales reached $16.1 million, demonstrating its growing contribution. The company's strategic focus on expanding sales efforts and achieving international approvals is key to maximizing these revenue streams.
| Revenue Stream | Description | 2024 Data/Outlook |
|---|---|---|
| NUPLAZID Net Sales | Primary revenue from Parkinson's disease psychosis treatment. | Continued strong performance and market demand. |
| DAYBUE Net Sales | Revenue from Rett syndrome treatment, a significant commercial opportunity. | Q1 2024 net sales: $16.1 million; expected acceleration with expanded reach. |
| Milestone Payments | Non-recurring revenue from partnerships (e.g., Neuren Pharmaceuticals). | Bolsters overall financial performance. |
| Asset Monetization | One-time revenue from strategic asset sales (e.g., PRV). | Example: $150 million from a Rare Pediatric Disease PRV sale. |
| Future Product Sales | Anticipated revenue from pipeline assets (ACP-101, ACP-204). | Expected mid-to-late 2020s contribution; targeting significant unmet needs. |
| International Sales | Revenue from expansion into markets outside North America, especially the EU. | Projected EU contribution of $100-$250 million annually within 3-5 years post-entry. |
Business Model Canvas Data Sources
The ACADIA Business Model Canvas is informed by comprehensive market research, internal financial data, and expert strategic analysis. This rigorous approach ensures each component reflects current market realities and our strategic objectives.
