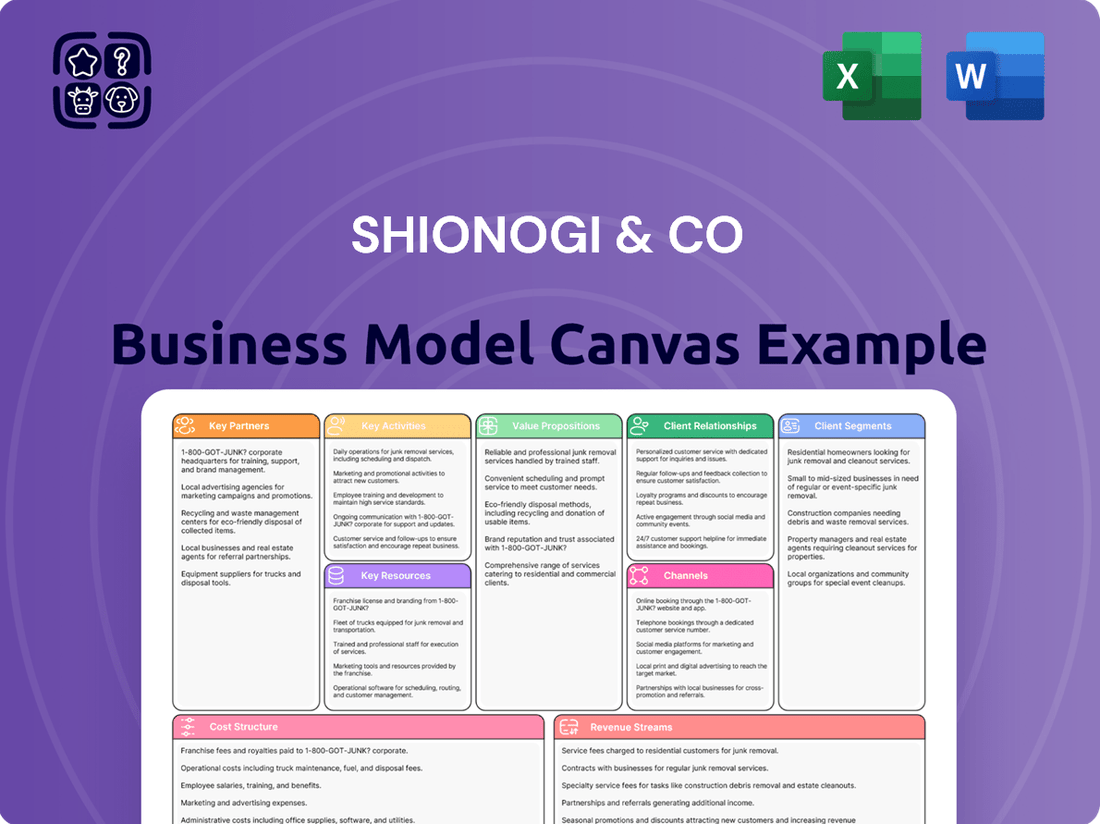
Shionogi & Co Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Shionogi & Co Bundle
Unlock the strategic blueprint behind Shionogi & Co's success with our comprehensive Business Model Canvas. This detailed analysis breaks down their value propositions, customer relationships, and revenue streams. Discover how they leverage key resources and activities to maintain their competitive edge in the pharmaceutical industry. Ideal for anyone seeking to understand the core mechanics of a thriving healthcare business.
Want to see exactly how Shionogi & Co operates and scales its business? Our full Business Model Canvas provides a detailed, section-by-section breakdown—perfect for benchmarking, strategic planning, or investor presentations. Download the complete version to gain actionable insights into their customer segments, cost structure, and key partnerships.
Partnerships
Shionogi actively collaborates with other pharmaceutical and biotech firms through co-development, licensing, and acquisition deals. These partnerships are crucial for Shionogi to broaden its product pipeline and extend its market access.
A notable collaboration for Shionogi is its work on HIV therapies with ViiV Healthcare. This strategic alliance aims to advance the development and commercialization of innovative treatments in a key therapeutic area.
Further strengthening its R&D and global footprint, Shionogi acquired Japan Tobacco's pharmaceutical business. This move also brought subsidiaries like Torii Pharmaceutical and Akros Pharma Inc. under Shionogi's umbrella, bolstering its capabilities.
Shionogi actively collaborates with academic and research institutions, recognizing their vital role in pioneering early-stage drug discovery and driving scientific progress. These partnerships are instrumental in accessing cutting-edge research and specialized expertise that complement Shionogi's internal capabilities. For instance, collaborations with universities like Osaka Metropolitan University focus on critical areas such as infectious disease research, including the pressing issue of antimicrobial resistance.
By leveraging external scientific knowledge, Shionogi aims to accelerate the development of novel therapeutic solutions and effectively tackle significant public health concerns. This strategic approach allows the company to tap into a broader pool of innovation, fostering a more dynamic and efficient research pipeline. Shionogi's commitment to these academic ties underscores its dedication to advancing medical science and improving patient outcomes globally.
Shionogi strategically collaborates with Contract Research Organizations (CROs) to accelerate its drug development pipeline, particularly in crucial clinical trial phases. For instance, in 2024, Shionogi continued its extensive work with various CROs to advance its late-stage clinical candidates in areas like infectious diseases and central nervous system disorders.
Complementing its research outsourcing, Shionogi also leverages Contract Manufacturing Organizations (CMOs) to ensure efficient and high-quality production of its pharmaceutical products. This allows the company to scale manufacturing operations effectively, meeting market demand promptly while maintaining stringent quality controls for its medicines.
These partnerships are vital for Shionogi's operational efficiency, enabling the company to optimize resource allocation by outsourcing specialized functions. By entrusting complex manufacturing and research processes to expert partners, Shionogi can focus its internal resources on core competencies like drug discovery and commercialization, driving innovation and market penetration.
Governmental and Non-Governmental Health Organizations
Shionogi actively partners with governmental health organizations like the Biomedical Advanced Research and Development Authority (BARDA) and non-governmental entities such as GARDP (Global Antibiotic Research and Development Partnership). These collaborations are crucial for tackling significant global health challenges, particularly infectious diseases and the growing threat of antimicrobial resistance (AMR). For instance, Shionogi's engagement with BARDA has supported the development of critical medical countermeasures, aligning with U.S. national security and public health priorities.
These vital partnerships often entail significant financial investments, joint research projects, and strategic initiatives aimed at improving access to essential medicines. A key focus is often on expanding healthcare reach into low- and middle-income countries, ensuring that life-saving treatments are available where they are most needed. Such efforts are fundamental to Shionogi's commitment to addressing unmet medical needs on a global scale.
- BARDA Collaboration: Supports the development of medical countermeasures, enhancing national health security.
- GARDP Partnership: Focuses on combating antimicrobial resistance (AMR) and expanding access to treatments.
- Global Access Initiatives: Aims to deliver essential medicines to underserved populations in low- and middle-income countries.
- Research and Development Funding: Leverages external investment to advance critical public health research programs.
Digital Health and AI Technology Partners
Shionogi actively collaborates with digital health and AI technology firms to advance its pipeline, particularly in areas like digital therapeutics and AI-powered drug discovery. This strategic approach is crucial for streamlining research and development processes. For instance, Shionogi's past joint venture with Ping An Insurance, which has since transitioned to a wholly-owned subsidiary for its China operations, highlights a commitment to leveraging digital platforms.
The company utilizes advanced data analytics platforms such as Snowflake and SAS Viya for robust analysis of real-world data (RWD). Furthermore, the integration of AI-SAS platforms for real-world evidence (RWE) collection and analysis demonstrably enhances efficiency. These partnerships are vital for accelerating Shionogi's ability to identify new therapeutic targets and optimize existing treatments, ultimately driving innovation in patient care.
- Digital Therapeutics Development: Partnering with tech companies to create digital tools that complement pharmaceutical treatments.
- AI-Driven Drug Discovery: Collaborating on platforms that use artificial intelligence to accelerate the identification and validation of drug candidates.
- RWD/RWE Analytics: Leveraging partnerships with data analytics providers like Snowflake and SAS for advanced insights from real-world data.
- Enhanced Research Efficiency: Utilizing AI and advanced analytics to improve the speed and accuracy of research and market analysis.
Shionogi's key partnerships extend to academic institutions, CROs, CMOs, and digital health firms. Collaborations with universities like Osaka Metropolitan University focus on infectious diseases. In 2024, Shionogi continued extensive work with CROs for clinical trials in infectious diseases and CNS disorders.
The company also partners with CMOs for efficient manufacturing and leverages digital health/AI firms for drug discovery and digital therapeutics. Partnerships with governmental bodies like BARDA and non-governmental organizations such as GARDP are crucial for addressing global health challenges, especially antimicrobial resistance.
| Partner Type | Focus Area | Example/Impact |
| Academic/Research Institutions | Early-stage drug discovery, infectious diseases | Osaka Metropolitan University (antimicrobial resistance) |
| CROs (Contract Research Organizations) | Clinical trial acceleration | Advancing late-stage candidates in infectious diseases and CNS (2024 focus) |
| CMOs (Contract Manufacturing Organizations) | Product manufacturing, quality control | Scaling production to meet market demand |
| Digital Health/AI Firms | Digital therapeutics, AI-driven drug discovery | AI-SAS platforms for RWE, Snowflake/SAS Viya for RWD analysis |
| Governmental/NGOs | Global health challenges, AMR | BARDA (medical countermeasures), GARDP (AMR, access) |
What is included in the product
A detailed, narrative-driven Shionogi & Co. Business Model Canvas, meticulously outlining their focus on unmet medical needs and their innovative drug development pipeline.
This canvas provides a deep dive into Shionogi's customer segments (patients, healthcare providers), value propositions (novel therapies, patient-centric solutions), and key partnerships.
Shionogi & Co.'s Business Model Canvas serves as a pain point reliever by offering a high-level, editable view that quickly identifies core components for strategic adaptation.
Activities
Shionogi's core activity is the intensive research and development of novel pharmaceutical products, with a strategic focus on critical therapeutic areas such as infectious diseases, pain management, and central nervous system (CNS) disorders.
This encompasses the entire drug development pipeline, from initial drug discovery and rigorous preclinical testing to the meticulous management of multiple phases of human clinical trials, all aimed at successfully bringing innovative therapies to patients.
In the fiscal year ending March 2024, Shionogi reported substantial investment in R&D, allocating approximately ¥110 billion, underscoring their commitment to innovation and pipeline expansion.
The company's R&D strategy balances the pursuit of entirely new therapeutic targets with the strategic life cycle management of their existing, successful drug compounds to ensure sustained growth and market relevance.
Shionogi & Co. directly manages the intricate manufacturing of its pharmaceutical products, diagnostic reagents, and medical devices. This hands-on approach is crucial for guaranteeing the highest quality and adherence to rigorous global regulatory standards. For instance, in fiscal year 2024, the company continued to invest in advanced manufacturing technologies to enhance efficiency and product integrity across its diverse portfolio.
The company’s manufacturing operations are characterized by complex production processes, requiring sophisticated supply chain management to ensure timely delivery and availability of essential medicines. Shionogi's commitment to stringent quality assurance is paramount, aiming to meet substantial market demand while prioritizing patient safety above all else.
Shionogi's sales and marketing efforts are crucial for getting its pharmaceutical products to patients worldwide. They employ dedicated sales teams who directly engage with doctors, hospitals, and pharmacies. In 2024, Shionogi continued its focus on promoting its key therapeutic areas, including infectious diseases and pain management, through various channels to ensure widespread availability of its innovations.
Regulatory Affairs and Compliance
Shionogi’s key activities heavily involve navigating intricate global regulatory landscapes. This means meticulously preparing and submitting new drug applications, securing market approvals, and consistently adhering to ever-changing healthcare regulations across numerous countries. In 2024, Shionogi continued its focus on obtaining approvals for its key products, particularly in the infectious disease and pain management areas, which are critical for its revenue streams. For instance, ongoing regulatory submissions for its COVID-19 oral antiviral, ensitrelvir (Xocova), in new markets remain a significant undertaking.
Maintaining compliance with these evolving regulations is paramount. This ensures Shionogi's pharmaceutical products can be legally marketed and distributed on a worldwide scale. The company dedicates substantial resources to regulatory affairs teams who monitor and interpret new guidelines, conduct post-market surveillance, and manage pharmacovigilance activities. This proactive approach is essential to mitigate risks and maintain the trust of healthcare providers and patients alike.
- Regulatory Submissions: Preparing and filing new drug applications (NDAs) and variations with global health authorities such as the FDA, EMA, and PMDA.
- Market Authorization: Obtaining and maintaining marketing authorizations for Shionogi's pharmaceutical products in key geographical regions.
- Compliance Monitoring: Ensuring adherence to Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and other relevant pharmaceutical regulations.
- Pharmacovigilance: Managing adverse event reporting and post-market safety surveillance to ensure product safety.
Strategic Investments and Acquisitions
Shionogi & Co. actively engages in strategic investments, in-licensing, and mergers and acquisitions to bolster its drug pipeline and expand into new therapeutic areas. This proactive approach is crucial for maintaining a competitive edge in the pharmaceutical industry. For instance, the company's commitment to growth was exemplified by its acquisition of Japan Tobacco's pharmaceutical business, a move designed to diversify its portfolio and enhance its market presence.
These strategic moves are not just about size; they are about acquiring novel technologies and promising drug candidates. By integrating external innovations, Shionogi can accelerate its research and development efforts and bring impactful treatments to patients more quickly. This strategy is vital for navigating the complex and rapidly evolving landscape of drug discovery and development.
- Pipeline Enhancement: Shionogi invests in companies and technologies that offer late-stage clinical assets or novel discovery platforms, aiming to fill gaps and strengthen its portfolio across key therapeutic areas like infectious diseases and pain management.
- Therapeutic Area Expansion: Acquisitions and in-licensing deals are strategically chosen to broaden Shionogi's reach into new or under-served medical fields, thereby diversifying revenue streams and reducing reliance on existing product lines.
- Technological Capabilities: The company seeks to acquire or partner with entities possessing cutting-edge research tools, manufacturing processes, or digital health solutions that can improve efficiency and innovation across its operations.
- Growth and Diversification: The acquisition of Japan Tobacco's pharmaceutical business in 2022, for example, was a significant step towards expanding Shionogi's footprint in Japan and diversifying its product offerings.
Shionogi's key activities center on the rigorous research and development of new medicines, especially for infectious diseases and pain. They manage the entire drug creation process, from initial discovery through clinical trials. In the fiscal year ending March 2024, R&D spending reached approximately ¥110 billion, reflecting a strong commitment to innovation.
Direct manufacturing of pharmaceuticals and medical devices is another core function, ensuring high quality and regulatory compliance. This includes investing in advanced production technologies. Sales and marketing teams actively promote Shionogi's products to healthcare professionals globally, focusing on key therapeutic areas to ensure patient access.
Navigating complex international regulations is crucial for market approval and ongoing compliance. Shionogi dedicates significant resources to regulatory affairs, managing submissions and post-market safety. The company actively pursues strategic investments, licensing, and acquisitions to enhance its drug pipeline and expand its therapeutic reach.
| Key Activity | Description | Fiscal Year 2024 Relevance |
| Research & Development | Discovery and development of novel pharmaceuticals. | ¥110 billion invested in R&D. Focus on infectious diseases, pain, CNS. |
| Manufacturing | Production of pharmaceuticals, diagnostic reagents, and medical devices. | Investment in advanced manufacturing technologies for quality and efficiency. |
| Sales & Marketing | Promoting products to healthcare professionals and ensuring market availability. | Continued focus on infectious diseases and pain management product promotion. |
| Regulatory Affairs | Securing market approvals and ensuring ongoing compliance. | Ongoing submissions for ensitrelvir (Xocova) in new markets. |
| Strategic Investments & M&A | Acquiring or partnering for pipeline enhancement and diversification. | Acquisition of Japan Tobacco's pharmaceutical business aimed at portfolio diversification. |
Preview Before You Purchase
Business Model Canvas
The Shionogi & Co Business Model Canvas you are previewing is the authentic document you will receive upon purchase. This is not a sample or mockup; it represents the entirety of the comprehensive analysis. Once your order is complete, you will gain full access to this exact, ready-to-use Business Model Canvas, allowing you to immediately leverage its insights for strategic planning.
Resources
Shionogi's intellectual property, particularly its robust patent portfolio, is a cornerstone of its business model. These patents cover innovative drug compounds, advanced manufacturing processes, and even diagnostic methods, safeguarding Shionogi's unique scientific advancements.
This extensive intellectual property protection is vital for maintaining a competitive edge in the pharmaceutical industry. It allows Shionogi to exclusively commercialize its novel therapies, directly driving revenue generation from its research and development efforts.
As of the fiscal year ending March 31, 2024, Shionogi reported substantial investments in research and development, underscoring the ongoing creation and protection of its intellectual capital. This commitment ensures a pipeline of innovative products secured by strong patent rights.
Shionogi's competitive edge is deeply rooted in its cadre of highly skilled researchers, scientists, and medical professionals. This intellectual capital is the engine driving their innovation in drug discovery, clinical development, and crucial medical affairs. Their combined expertise is indispensable for pinpointing critical unmet medical needs and formulating groundbreaking therapeutic solutions.
In 2024, Shionogi continued to invest heavily in its human capital, a testament to the value placed on scientific and medical expertise. The company's commitment to R&D, which forms the backbone of its business model, directly leverages the deep knowledge base of its personnel. This focus ensures Shionogi remains at the forefront of pharmaceutical advancements, translating scientific understanding into tangible patient benefits.
Shionogi & Co operates state-of-the-art manufacturing facilities, crucial for producing its pharmaceutical products to the highest quality standards. These plants are designed for efficiency and compliance with stringent global regulations, ensuring the integrity of every batch.
A robust global supply chain network complements these facilities, enabling Shionogi to deliver its medicines efficiently to patients worldwide. This network is vital for managing raw material sourcing, production logistics, and final product distribution across diverse markets.
In fiscal year 2024, Shionogi continued to invest in its manufacturing capabilities, aiming to enhance production capacity and streamline operations. For example, the company's R&D pipeline, as of early 2025, includes several promising late-stage candidates, necessitating scalable manufacturing solutions.
The company's commitment to a resilient supply chain was evident in its proactive management of potential disruptions, ensuring consistent product availability for critical therapies. This focus is particularly important given the global demand for innovative treatments in areas like infectious diseases and pain management, Shionogi's core therapeutic areas.
Financial Capital
Shionogi & Co. relies heavily on substantial financial capital to fuel its core operations. This includes significant investments in research and development, which are critical for discovering and bringing new pharmaceutical products to market. For instance, in the fiscal year ending March 2024, Shionogi reported R&D expenses of approximately ¥114.3 billion (around $770 million USD, based on an approximate exchange rate).
This financial backing is also essential for conducting the rigorous and lengthy clinical trials required to gain regulatory approval for new drugs. Beyond R&D, Shionogi's financial resources support its global manufacturing capabilities and extensive marketing efforts to ensure its products reach patients. The company's robust financial health, evidenced by its strong balance sheet and consistent revenue streams, empowers it to pursue strategic acquisitions and partnerships that can accelerate growth and expand its therapeutic areas.
- Research & Development Funding: Shionogi allocated ¥114.3 billion to R&D in FY2024, underscoring its commitment to innovation.
- Clinical Trial Investment: Significant capital is dedicated to the costly and time-consuming process of clinical trials.
- Operational Expenses: Financial resources are vital for manufacturing, supply chain management, and global marketing initiatives.
- Strategic Growth: Financial strength enables Shionogi to pursue mergers, acquisitions, and investments in high-potential projects.
Clinical Data and Real-World Evidence (RWE)
Shionogi's accumulated clinical trial data and real-world evidence (RWE) are critical assets. This data demonstrates product efficacy and safety, which is essential for gaining regulatory approval and shaping how healthcare professionals use their treatments. By analyzing this evidence, Shionogi can further refine its product offerings and identify new therapeutic opportunities.
The company utilizes sophisticated data analysis environments, often cloud-based, to process and interpret this vast amount of information. This technological infrastructure allows Shionogi to generate high-quality, actionable insights from their clinical and real-world data. For instance, in 2024, Shionogi continued to invest in these advanced analytics capabilities to better understand patient outcomes and treatment patterns.
- Clinical Trial Data: Forms the bedrock for demonstrating safety and efficacy to regulatory bodies like the FDA and EMA.
- Real-World Evidence (RWE): Provides insights into product performance in routine clinical practice, complementing trial data.
- Data Analysis Environments: Shionogi employs advanced platforms, including cloud solutions, to process and analyze large datasets efficiently.
- Evidence Generation: The focus is on creating high-quality evidence to support product value and inform medical decision-making.
Shionogi's key resources are multifaceted, encompassing its strong intellectual property, a highly skilled workforce, advanced manufacturing facilities, a robust global supply chain, substantial financial capital, and a growing repository of clinical and real-world data. These elements collectively form the foundation upon which the company builds its innovative pharmaceutical business.
The company's intellectual property, particularly its patent portfolio, safeguards its novel drug compounds and manufacturing processes, ensuring a competitive advantage. This is complemented by the expertise of its researchers and scientists, who drive drug discovery and development. Shionogi also operates state-of-the-art manufacturing plants and a global supply chain network to ensure efficient product delivery.
Financially, Shionogi demonstrates a strong commitment to R&D, investing ¥114.3 billion in FY2024 to fuel innovation and clinical trials. This financial strength also supports operational expenses and strategic growth initiatives. Furthermore, the company leverages accumulated clinical trial data and real-world evidence, analyzed through advanced data environments, to demonstrate product value and inform medical practice.
| Key Resource | Description | FY2024 Relevance |
|---|---|---|
| Intellectual Property | Patents on drug compounds, manufacturing processes | Secures competitive edge, drives revenue |
| Human Capital | Skilled researchers, scientists, medical professionals | Drives innovation, identifies unmet medical needs |
| Physical Assets | State-of-the-art manufacturing facilities, global supply chain | Ensures quality production and efficient delivery |
| Financial Capital | Investment in R&D, clinical trials, operations | ¥114.3 billion R&D spend; supports growth |
| Data & Analytics | Clinical trial data, real-world evidence, analysis platforms | Demonstrates efficacy, informs medical decisions |
Value Propositions
Shionogi is dedicated to developing groundbreaking treatments for diseases where current options are insufficient. Their focus areas include challenging infectious diseases, such as tackling multi-drug resistant bacteria and developing antivirals for COVID-19 and influenza. By investing heavily in research, Shionogi aims to deliver therapies that truly make a difference in patients' lives, particularly in areas like central nervous system disorders.
Shionogi & Co. is dedicated to providing pharmaceutical products that are not only effective but also consistently reliable. This unwavering focus on quality is paramount for ensuring patient safety and fostering strong trust with both medical practitioners and the regulatory bodies that oversee the industry. Their portfolio extends beyond just medications to include diagnostic reagents and essential medical devices, all adhering to stringent quality standards.
In 2024, Shionogi continued its commitment to product integrity, a cornerstone of its business model. For instance, their ongoing investment in research and development, which often exceeds 20% of net sales, directly supports the creation of high-quality, reliable pharmaceuticals. This financial commitment underscores their strategy to maintain a competitive edge through superior product development and manufacturing processes.
Shionogi actively addresses critical global health threats, particularly antimicrobial resistance (AMR). By focusing on developing novel antibacterial agents, Shionogi aims to combat the growing challenge of drug-resistant infections that endanger public health worldwide. For instance, Shionogi's commitment to AMR research is demonstrated by its pipeline and past successful product launches, contributing to a more resilient global healthcare infrastructure.
Improved Patient Quality of Life
Shionogi is dedicated to improving patient well-being by developing innovative treatments for conditions such as chronic pain, sleep disorders, and hearing loss. Their focus is on creating therapies that not only manage symptoms but also restore daily functioning and overall life satisfaction for individuals facing these challenges.
The company's commitment to enhancing patient quality of life is reflected in its pipeline and existing product portfolio. For instance, Shionogi's work in pain management aims to provide alternatives to opioids, addressing a significant public health concern. In 2024, Shionogi continued to invest heavily in research and development, with a significant portion allocated to these patient-centric therapeutic areas, underscoring their strategic priority.
- Alleviation of Chronic Pain: Developing non-opioid pain relievers to improve daily activities and reduce suffering.
- Restoration of Sleep Quality: Creating treatments for sleep disorders that promote restful sleep and daytime alertness.
- Improvement of Hearing Function: Researching novel therapies to address hearing impairment and its impact on communication and social interaction.
- Enhanced Daily Functioning: Enabling patients to engage more fully in life by managing debilitating symptoms.
Comprehensive Healthcare Solutions
Shionogi's commitment to comprehensive healthcare extends beyond traditional pharmaceuticals. They are actively developing and integrating digital therapeutics, aiming to provide more complete patient care pathways. This strategic pivot allows them to offer enhanced disease management and robust patient support, leveraging technology to improve outcomes.
This expansion into digital solutions is a significant move for Shionogi. For instance, by early 2024, the company had already invested in and partnered with several digital health startups focused on areas like chronic condition management. These initiatives are designed to complement their existing drug portfolios, creating a more holistic approach to patient well-being.
- Holistic Patient Care: Moving beyond single-molecule solutions to integrated health services.
- Digital Therapeutics Integration: Leveraging technology for enhanced disease management and patient support.
- Strategic Partnerships: Collaborating with digital health innovators to expand service offerings.
- Improved Health Outcomes: Aiming for more comprehensive patient support and better treatment efficacy.
Shionogi's value proposition centers on delivering innovative treatments for unmet medical needs, particularly in challenging infectious diseases and central nervous system disorders. They focus on high-quality, reliable pharmaceuticals, diagnostic reagents, and medical devices, ensuring patient safety and trust. Their commitment extends to improving patient quality of life through pain management alternatives to opioids and treatments for sleep and hearing disorders, aiming to restore daily functioning and satisfaction.
Furthermore, Shionogi integrates digital therapeutics to offer holistic patient care, enhancing disease management and support through strategic partnerships with digital health innovators. This dual approach of pharmaceutical innovation and digital integration aims for superior health outcomes and comprehensive patient well-being.
| Key Value Proposition Area | Focus | 2024 Impact/Investment Example |
|---|---|---|
| Therapeutic Innovation | Unmet medical needs (infectious diseases, CNS) | Continued R&D investment, often exceeding 20% of net sales, supporting novel antibacterial agents and CNS therapies. |
| Product Quality & Reliability | Patient safety, trust with practitioners/regulators | Stringent quality standards across pharmaceuticals, diagnostic reagents, and medical devices. |
| Patient Quality of Life | Pain management, sleep, hearing disorders | Development of non-opioid pain relievers; investment in therapies to improve sleep and hearing function. |
| Digital Health Integration | Holistic patient care, enhanced management | Partnerships with digital health startups for chronic condition management, complementing drug portfolios. |
Customer Relationships
Shionogi cultivates robust relationships with healthcare professionals (HCPs), including physicians and specialists, primarily through comprehensive medical education programs and scientific symposia. These initiatives ensure HCPs are thoroughly informed about Shionogi's innovative treatments and their appropriate application. By the end of fiscal year 2024, Shionogi reported a significant increase in engagement across its key therapeutic areas, with over 5,000 educational events conducted globally, reaching more than 150,000 HCPs.
A dedicated sales force plays a crucial role in fostering these direct interactions, providing essential product information and clinical support. This direct engagement is vital for empowering HCPs to make well-informed prescribing decisions that benefit patient care. Shionogi's sales representatives conducted over 300,000 individual detailing sessions in 2024, focusing on new data and patient-centric approaches.
Shionogi actively collaborates with patient advocacy groups, recognizing their vital role in understanding patient needs and fostering trust. These partnerships are crucial for Shionogi to gain insights and ensure their efforts align with the real-world experiences of individuals managing various health conditions.
The company’s commitment to patient well-being is demonstrated through its support programs. These initiatives aim to provide patients with essential information, resources, and access to treatments, going beyond just the transactional aspect of selling medication.
For instance, in 2024, Shionogi continued to invest in patient support services, with a focus on areas like infectious diseases and pain management, where patient education and ongoing support are particularly critical. This holistic approach underscores their dedication to improving patient outcomes.
Shionogi & Co. actively cultivates professional relationships with key institutional entities like hospitals, clinics, and government health bodies. These organizations are vital as they represent major purchasers and influencers in the prescription of Shionogi's pharmaceutical products.
These crucial relationships are managed through direct sales channels and formal procurement agreements. For instance, Shionogi's engagement with the Japanese Ministry of Health, Labour and Welfare for vaccine procurement highlights this institutional tie-in.
Beyond transactional interactions, Shionogi also engages in collaborative efforts with these institutions on public health initiatives. This collaborative approach aims to address broader health challenges and strengthen Shionogi's role within the healthcare ecosystem.
Research Collaborations and Partnerships
Shionogi & Co. cultivates crucial research collaborations and partnerships by strategically engaging with academic institutions and industry peers. These relationships are formalized through various avenues, including dedicated strategic alliances, the formation of joint ventures, and the execution of comprehensive licensing agreements. This approach allows Shionogi to leverage external expertise and resources, accelerating the drug development pipeline and enhancing commercialization efforts.
The foundation of these partnerships rests on a bedrock of shared scientific curiosity and aligned objectives. Both parties are driven by the common goal of advancing novel therapeutics from discovery through to market. In 2024, Shionogi continued to actively pursue such collaborations, recognizing their indispensable role in navigating the complex landscape of pharmaceutical innovation. For instance, their ongoing work with leading universities globally exemplifies this commitment, focusing on early-stage research in areas like infectious diseases and oncology.
- Strategic Alliances: Formal agreements to pool resources and expertise for specific research projects, often leading to shared intellectual property.
- Joint Ventures: Establishing new entities with partners to co-develop and commercialize specific drug candidates, sharing risks and rewards.
- Licensing Agreements: Acquiring rights to promising technologies or compounds from external entities, or out-licensing Shionogi's own innovations.
- Mutual Scientific Interest: Collaborations are driven by a genuine shared passion for scientific advancement and the potential to address unmet medical needs.
Investor Relations and Shareholder Dialogue
Shionogi & Co. prioritizes clear and consistent communication with its investors and shareholders. This is achieved through regular financial reports, dedicated investor meetings, and various investor relations (IR) events designed to keep stakeholders fully informed.
The company's commitment to transparency ensures that financial stakeholders have access to up-to-date information regarding Shionogi's performance, strategic direction, and future growth prospects. For instance, during fiscal year 2024, Shionogi actively engaged with the investment community, holding numerous calls and presentations to discuss its pipeline advancements and financial results.
- Financial Reports: Shionogi regularly publishes quarterly and annual financial statements, providing detailed insights into revenue, profitability, and R&D investments.
- Investor Meetings and Calls: The company hosts regular meetings and conference calls with analysts and investors to discuss financial performance and strategic updates.
- IR Events: Shionogi participates in industry conferences and hosts its own investor days to showcase its research, development progress, and business strategy.
- Shareholder Dialogue: Direct engagement through these channels fosters a strong relationship and understanding between Shionogi and its shareholder base.
Shionogi & Co. focuses on building strong relationships with healthcare professionals through extensive medical education and scientific events, aiming to inform them about innovative treatments. The company also relies on a dedicated sales force for direct engagement and support, facilitating informed prescribing decisions.
Furthermore, Shionogi partners with patient advocacy groups to understand patient needs and fosters trust through support programs that enhance treatment access and information. These relationships are crucial for aligning company efforts with real-world patient experiences and improving overall outcomes.
Shionogi also cultivates key relationships with institutional entities like hospitals and government health bodies, which are vital for product prescription and procurement. Collaborative efforts on public health initiatives further solidify its role within the broader healthcare ecosystem.
Research collaborations with academic institutions and industry peers, formalized through alliances, joint ventures, and licensing agreements, accelerate drug development and commercialization. These partnerships are driven by shared scientific goals, as exemplified by Shionogi's ongoing work with global universities in 2024.
Channels
Shionogi & Co. relies on a robust network of pharmaceutical wholesalers and distributors to get its medicines to patients. This is a critical part of their strategy for broad market reach. These intermediaries, like AmerisourceBergen or Cardinal Health in the US, ensure that Shionogi's products are available in pharmacies, hospitals, and clinics worldwide.
This channel strategy is key for widespread product availability and market penetration. In 2024, the global pharmaceutical distribution market was valued at over $1.3 trillion, highlighting the scale and importance of these partnerships for companies like Shionogi.
Shionogi & Co. leverages a dedicated direct sales force as a critical channel to connect with healthcare professionals, hospitals, and influential medical experts. This approach is essential for detailed product education and fostering strong relationships, particularly for sophisticated treatments.
In 2023, Shionogi reported significant revenue, with its direct sales force playing a key role in driving the adoption of its innovative pharmaceutical products, especially in specialized medical fields.
This direct engagement allows for in-depth discussions about Shionogi's offerings, addressing the unique needs of prescribers and ensuring accurate understanding of product benefits and applications.
The company's investment in a specialized sales team underscores its commitment to building trust and providing valuable support within the medical community, directly impacting market penetration and sales performance.
Hospitals and clinics are a crucial direct channel for Shionogi, especially for its specialized treatments. Many of these products, like those targeting infectious diseases or central nervous system disorders, are administered within these healthcare settings, making them key points of product utilization.
In 2024, the global hospital market continued its robust growth, projected to reach over $11 trillion by the end of the year. This expansion directly benefits pharmaceutical companies like Shionogi, as increased hospital admissions and treatments translate to higher demand for their medications.
Shionogi's focus on areas such as anti-infectives means that hospitals are primary customers. For instance, the ongoing need for effective treatments against evolving bacterial resistance patterns ensures a consistent demand for Shionogi's innovative antibiotics within hospital formularies and treatment protocols.
Pharmacies (Retail and Hospital)
Pharmacies, both retail and hospital-based, are crucial channels for Shionogi & Co. They act as the direct interface for dispensing prescription and over-the-counter medications to patients, ensuring Shionogi’s products reach the end consumer. By partnering with extensive pharmacy networks, Shionogi enhances the accessibility and availability of its innovative treatments.
In 2024, Shionogi continued to strengthen its relationships with major pharmacy chains and independent pharmacies across its key markets. This focus is vital for timely patient access, especially for Shionogi's specialized therapies. The company aims to optimize inventory management and reduce dispensing times through these collaborations.
- Direct Patient Access: Pharmacies are the primary touchpoint for patients to receive Shionogi's prescription and OTC drugs, ensuring treatment continuity.
- Network Partnerships: Shionogi collaborates with broad pharmacy networks to maximize the availability of its medicines, facilitating wider patient reach.
- Logistical Integration: Working with pharmacies helps streamline the supply chain, ensuring efficient delivery and inventory management for Shionogi's product portfolio.
Digital Platforms and Healthcare Technology Integrations
Shionogi leverages digital platforms as a crucial channel for disseminating vital information to healthcare professionals and patients. This includes educational content and updates on their therapeutic areas. In 2024, the company continued to expand its digital footprint, aiming to reach a wider audience with specialized medical knowledge.
The integration of Shionogi's offerings with existing healthtech platforms is a key strategy for enhancing patient outcomes. This synergy allows for improved patient engagement and can significantly boost treatment adherence by providing accessible tools and reminders. By connecting with these platforms, Shionogi aims to create a more seamless patient journey.
- Information Dissemination: Shionogi utilizes digital channels to share scientific data and treatment guidelines with healthcare providers.
- Medical Education: Online webinars and digital resources are employed to educate medical professionals on new research and therapies.
- Digital Therapeutics Potential: The company is exploring the role of digital therapeutics, which could be distributed via these platforms.
- Healthtech Integration: Partnerships with healthtech companies facilitate better patient monitoring and support.
Shionogi & Co. utilizes a multi-faceted channel strategy to ensure its innovative pharmaceutical products reach patients effectively. This includes established relationships with wholesalers and distributors for broad market access, a direct sales force for targeted engagement with healthcare professionals, and direct sales to hospitals and clinics, particularly for specialized treatments.
Pharmacies, both retail and hospital-based, serve as a critical final touchpoint for dispensing medications to patients, ensuring accessibility. Complementing these physical channels, Shionogi also leverages digital platforms for information dissemination and engages with healthtech integrations to enhance patient outcomes and adherence.
| Channel | Key Function | 2024 Relevance/Data Point |
|---|---|---|
| Wholesalers & Distributors | Broad market reach, product availability | Global pharmaceutical distribution market valued over $1.3 trillion. |
| Direct Sales Force | Product education, relationship building with HCPs | Key driver for adoption of specialized treatments. |
| Hospitals & Clinics | Direct administration of specialized treatments | Global hospital market projected to exceed $11 trillion. |
| Pharmacies (Retail & Hospital) | Dispensing to end consumers, patient access | Strengthened partnerships with major pharmacy chains. |
| Digital Platforms & Healthtech | Information dissemination, patient engagement, adherence | Expansion of digital footprint, exploration of digital therapeutics. |
Customer Segments
Shionogi's customer segment of patients with infectious diseases encompasses individuals battling a range of bacterial and viral infections. This includes those afflicted by challenging gram-negative bacterial infections, a persistent global health concern, as well as influenza and the ongoing threat of COVID-19. For these patients, Shionogi is dedicated to developing and delivering effective antiviral and antibacterial treatments designed to combat these specific pathogens and improve health outcomes.
Shionogi's business model actively targets patients grappling with Central Nervous System (CNS) disorders and chronic pain. This significant customer segment includes individuals suffering from conditions like ADHD, sleep disorders, and various neurological ailments. The company's focus is on developing novel therapies that effectively manage symptoms and enhance the overall well-being of these patients.
Healthcare professionals, including physicians, specialists like infectious disease experts, neurologists, and psychiatrists, along with pharmacists, form a cornerstone customer segment for Shionogi. Their role in prescribing, recommending, and dispensing Shionogi's pharmaceutical products directly influences patient access and treatment outcomes.
The adoption and deep understanding of Shionogi's innovative medicines by these professionals are paramount. For instance, in 2024, Shionogi continued to focus on educating these key opinion leaders and prescribers on the efficacy and safety profiles of its portfolio, particularly in areas like infectious diseases and central nervous system disorders.
Hospitals, Clinics, and Diagnostic Laboratories
Hospitals, clinics, and diagnostic laboratories represent Shionogi's core organizational customer base, actively purchasing its pharmaceutical products, diagnostic reagents, and medical devices. These healthcare providers are critical for administering patient care and performing essential diagnostic procedures, directly impacting Shionogi's revenue streams and market penetration within the healthcare ecosystem.
These institutions rely on Shionogi for treatments and diagnostic tools that are fundamental to patient outcomes. For instance, Shionogi's portfolio includes vital anti-infectives and pain management solutions that are frequently administered in inpatient and outpatient settings. The demand from these segments directly reflects the ongoing need for effective medical interventions.
In 2024, the global hospital and clinic market continues to demonstrate robust growth, driven by an aging population and increasing healthcare expenditure. Diagnostic laboratories, too, are expanding, fueled by advancements in testing technologies and a greater emphasis on early disease detection. Shionogi's engagement with these segments is therefore strategically vital for sustained business development.
Key aspects of Shionogi's relationship with these customer segments include:
- Product Procurement: These entities are the primary purchasers of Shionogi's prescription drugs and over-the-counter medications.
- Diagnostic Solutions: Hospitals and labs utilize Shionogi's reagents and equipment for various diagnostic tests, crucial for treatment planning.
- Clinical Partnerships: Collaboration with these institutions often facilitates clinical trials and the adoption of new Shionogi innovations.
- Market Access: Their widespread presence ensures broad access to Shionogi's therapeutic offerings across diverse patient populations.
Government Health Agencies and Public Health Organizations
Government health agencies and public health organizations are crucial customer segments for Shionogi & Co. These entities often procure medicines for national stockpiles, a strategy significantly highlighted during the COVID-19 pandemic for treatments and vaccines. For instance, in 2024, many governments continued to invest in pandemic preparedness, securing diverse therapeutic options.
Furthermore, these organizations are key partners in addressing major public health threats, such as antimicrobial resistance (AMR). Shionogi's engagement with such bodies contributes to the development and implementation of broader health policies aimed at combating AMR and other infectious diseases. Their procurement decisions can significantly impact the availability and accessibility of essential medicines globally.
- Strategic Stockpiling: Governments procure Shionogi’s products for national health security, particularly for infectious disease threats.
- Public Health Initiatives: Collaboration on programs to combat AMR and other public health challenges.
- Policy Influence: Engaging with these bodies to shape health policies and ensure access to innovative treatments.
- Procurement Power: These organizations represent significant purchasing volume for Shionogi's portfolio, especially in critical health areas.
Shionogi's customer segments are diverse, encompassing patients requiring treatments for infectious diseases and central nervous system disorders. Healthcare professionals, including physicians and pharmacists, are crucial as they prescribe and recommend Shionogi's products. Hospitals, clinics, and diagnostic laboratories form a key organizational base, purchasing pharmaceuticals and diagnostic tools.
Government health agencies and public health organizations are also vital, procuring medicines for national stockpiles and partnering on public health initiatives like combating antimicrobial resistance. In 2024, Shionogi continued to focus on these segments, recognizing their pivotal role in market access and public health impact.
| Customer Segment | Key Role | 2024 Focus/Activity |
|---|---|---|
| Patients | End-users of Shionogi's therapies for infectious diseases and CNS disorders. | Continued development of innovative treatments to address unmet medical needs. |
| Healthcare Professionals | Prescribers, influencers, and administrators of Shionogi's medicines. | Education on product efficacy, safety, and new therapeutic advancements. |
| Hospitals & Clinics | Purchasers of pharmaceuticals, distributors of treatments to patients. | Ensuring availability of critical anti-infectives and pain management solutions. |
| Government Agencies | Procurement for public health, partners in health policy. | Engagement on pandemic preparedness and antimicrobial resistance strategies. |
Cost Structure
Research and Development (R&D) is a major cost driver for Shionogi & Co. In fiscal year 2023, Shionogi reported R&D expenses of approximately ¥144.5 billion (around $960 million USD at current exchange rates). This significant investment fuels the discovery of new medicines, preclinical studies, and extensive clinical trials necessary to bring innovative treatments to market.
These R&D expenditures are vital for Shionogi's long-term growth, as they directly contribute to building its future product pipeline and maintaining a competitive edge in the pharmaceutical industry. The company's commitment to R&D underscores its strategy of developing novel therapies for unmet medical needs.
Shionogi & Co. faces significant manufacturing and production costs, encompassing everything from sourcing raw materials to the final packaging of its pharmaceutical products, diagnostic reagents, and medical devices. These expenses are crucial to bringing their innovations to market.
In fiscal year 2024, Shionogi reported substantial investments in its manufacturing infrastructure and processes. For instance, the cost of goods sold (COGS) for their pharmaceutical segment alone represented a considerable portion of their revenue, reflecting the complex and highly regulated nature of drug production.
Key cost drivers include the procurement of specialized active pharmaceutical ingredients (APIs), which can be expensive and subject to supply chain volatility. Labor costs for skilled scientists, technicians, and production staff are also a major factor, alongside the ongoing maintenance and upgrades of state-of-the-art manufacturing facilities to ensure compliance with stringent global quality standards.
The company's commitment to quality control, involving rigorous testing at multiple stages of production, adds another layer of expense. These comprehensive quality assurance measures are vital for patient safety and regulatory approval, contributing to the overall manufacturing cost structure.
Shionogi & Co's Sales, General, and Administrative (SG&A) expenses encompass crucial operational costs like marketing, sales force activities, and distribution networks. These also include essential corporate functions such as executive and employee salaries, office rent, and legal counsel. For the fiscal year ending March 31, 2024, Shionogi reported SG&A expenses of ¥172.3 billion, a slight increase from the previous year, underscoring the investment in market reach and operational efficiency.
Regulatory and Compliance Costs
Shionogi & Co. faces substantial regulatory and compliance costs to navigate the complex global landscape of pharmaceutical development and sales. These expenses are crucial for ensuring drug safety, efficacy, and adherence to varying international standards. Meeting these requirements is non-negotiable for market access and maintaining public trust.
Key components of these costs include:
- Regulatory Submission Fees: Payments to health authorities like the FDA (USA), EMA (Europe), and PMDA (Japan) for reviewing new drug applications and subsequent variations.
- Compliance Audits and Quality Management: Ongoing investment in internal and external audits, robust quality management systems (QMS), and personnel training to meet Good Manufacturing Practice (GMP) and Good Clinical Practice (GCP) standards.
- Post-Market Surveillance: Costs associated with pharmacovigilance, monitoring drug performance and safety after approval, including adverse event reporting and risk management plans.
- Regional Compliance Adaptation: Expenses incurred in tailoring product information, labeling, and marketing materials to comply with specific regulations in each target market.
For instance, the pharmaceutical industry's overall spending on regulatory affairs and compliance is a significant operational expenditure. While specific figures for Shionogi are proprietary, industry benchmarks indicate that companies can spend millions annually per major market just on submission fees and maintaining compliance infrastructure, with post-market activities adding further to this burden.
Acquisition and Partnership Costs
Shionogi & Co incurs significant expenses through strategic acquisitions and partnership agreements to bolster its product pipeline and market reach. These costs are vital for expanding the company's capabilities and diversifying its therapeutic areas. For instance, in-licensing deals and upfront payments for new drug candidates represent a substantial portion of this cost structure, enabling access to innovative research and development.
Milestone payments, tied to the successful progression of partnered assets through clinical trials and regulatory approvals, also contribute to these acquisition and partnership costs. These variable payments ensure that Shionogi only incurs further expense as a drug demonstrates increasing value and potential. This approach allows for strategic resource allocation, focusing capital on promising opportunities.
- Acquisition Expenses: Costs associated with purchasing other companies or significant stakes in them to gain access to their technologies or product portfolios.
- In-Licensing Fees: Payments made to acquire the rights to develop and commercialize drugs or technologies from external partners.
- Upfront Payments: Initial fees paid to partners at the commencement of a collaboration or licensing agreement.
- Milestone Payments: Additional payments made to partners upon the achievement of specific predetermined goals, such as successful clinical trial phases or regulatory approvals.
Shionogi & Co.'s cost structure is heavily influenced by its research and development (R&D) investments, manufacturing operations, and sales, general, and administrative (SG&A) expenses.
In fiscal year 2024, R&D expenses were a significant driver, alongside substantial manufacturing costs and SG&A which stood at ¥172.3 billion. These expenditures are critical for innovation, production, and market penetration.
The company also allocates resources to regulatory compliance and strategic acquisitions, which are essential for long-term growth and market access.
Key cost components for Shionogi & Co.:
| Cost Component | Fiscal Year 2023/2024 Significance | Key Drivers |
| Research & Development (R&D) | ¥144.5 billion (FY2023) | New drug discovery, clinical trials, innovation |
| Manufacturing & Production | Significant portion of COGS | Raw materials, skilled labor, quality control, facilities |
| Sales, General & Administrative (SG&A) | ¥172.3 billion (FY2024) | Marketing, sales force, distribution, corporate functions |
| Regulatory & Compliance | Ongoing investment | Submission fees, audits, pharmacovigilance, market adaptation |
| Acquisitions & Partnerships | Strategic investment | In-licensing, upfront payments, milestone payments |
Revenue Streams
Shionogi's core revenue generation stems from the sales of its prescription pharmaceutical products. This is particularly true for their key therapeutic areas, which prominently feature treatments for infectious diseases and central nervous system (CNS) disorders.
Products like Fetroja, Xocova, and Xofluza are significant contributors to this revenue stream. These are medicines prescribed by doctors, meaning their sales are directly tied to patient treatment and healthcare provider adoption.
For the fiscal year ending March 2024, Shionogi reported net sales from pharmaceutical products reaching approximately ¥323.7 billion. This highlights the substantial impact of their ethical drug portfolio on the company's overall financial performance.
The sales figures for these prescription drugs are a direct reflection of their market penetration and the ongoing demand for effective treatments in their targeted disease areas.
Shionogi & Co. significantly leverages its intellectual property through royalties and licensing, generating a consistent revenue stream. A prime example is their collaboration with ViiV Healthcare on HIV treatments, which yields substantial royalty payments. This strategy diversifies income and reduces reliance on direct product sales.
Shionogi & Co. diversifies its revenue beyond pharmaceuticals through the sale of diagnostic reagents and medical devices. This strategic approach bolsters its financial stability and market presence.
In the fiscal year ending March 2024, Shionogi reported significant contributions from its diverse product lines, showcasing the impact of its diagnostic and medical device segments on its overall revenue. These sales underscore the company's commitment to a comprehensive healthcare solutions model.
Milestone Payments from Partnerships
Shionogi & Co also earns revenue through milestone payments from its collaboration partners. These payments are triggered when specific development, regulatory, or commercialization goals are met for drugs developed jointly. For instance, in 2024, Shionogi reported significant progress in several partnered programs, with potential for substantial milestone receipts as these projects advance.
These milestone payments are a crucial component of Shionogi's revenue diversification strategy, providing upfront capital and reflecting the successful progression of their research and development pipeline.
- Milestone Payments: Revenue earned when partnered drug development targets are achieved.
- Progress-Based: Payments are tied to specific development, regulatory, or commercialization achievements.
- Cash Infusion: Provides upfront capital and supports ongoing R&D efforts.
- 2024 Relevance: Key driver for Shionogi’s financial performance in the current year due to active collaborations.
Over-the-Counter (OTC) Product Sales
Shionogi & Co leverages over-the-counter (OTC) product sales as a supplementary revenue stream, diversifying its business beyond prescription pharmaceuticals. This segment allows for direct consumer engagement and builds brand familiarity in the healthcare space. In fiscal year 2023, Shionogi reported ¥13.7 billion (approximately $92 million USD at current exchange rates) in revenue from its Consumer Healthcare business, which includes OTC products.
- OTC segment contributes to revenue diversification.
- Enhances consumer brand recognition and market access.
- Fiscal year 2023 revenue from Consumer Healthcare was ¥13.7 billion.
- OTC products offer a different distribution channel compared to prescriptions.
Shionogi's revenue is primarily driven by prescription pharmaceutical sales, particularly in infectious diseases and CNS. For the fiscal year ending March 2024, pharmaceutical net sales reached approximately ¥323.7 billion, underscoring the significance of their ethical drug portfolio.
Beyond direct sales, Shionogi generates income through royalties and licensing agreements, such as those with ViiV Healthcare for HIV treatments. The company also diversifies with sales of diagnostic reagents and medical devices, contributing to financial stability.
Milestone payments from collaborations provide further revenue, linked to achieving development and regulatory goals. In fiscal year 2023, Shionogi's Consumer Healthcare business, including OTC products, reported ¥13.7 billion in revenue.
| Revenue Stream | Description | Fiscal Year Ending March 2024 (Approximate) |
|---|---|---|
| Pharmaceutical Sales | Sales of prescription drugs, notably in infectious diseases and CNS. | ¥323.7 billion (Net Sales from Pharmaceutical Products) |
| Royalties & Licensing | Income from intellectual property shared with partners. | (Specific data not provided for FY24, but significant contributor) |
| Diagnostic Reagents & Medical Devices | Sales from non-pharmaceutical healthcare products. | (Specific data not provided for FY24, but contributes to diversification) |
| Milestone Payments | Payments received upon achieving development or regulatory milestones in partnerships. | (Key driver for FY24, reflecting pipeline progress) |
| Consumer Healthcare (OTC) | Revenue from over-the-counter products. | ¥13.7 billion (Fiscal Year 2023) |
Business Model Canvas Data Sources
The Shionogi & Co. Business Model Canvas is built using a blend of internal financial reports, patent filings, and R&D investment data. These sources provide a robust foundation for understanding the company's current operations and future strategic direction.
