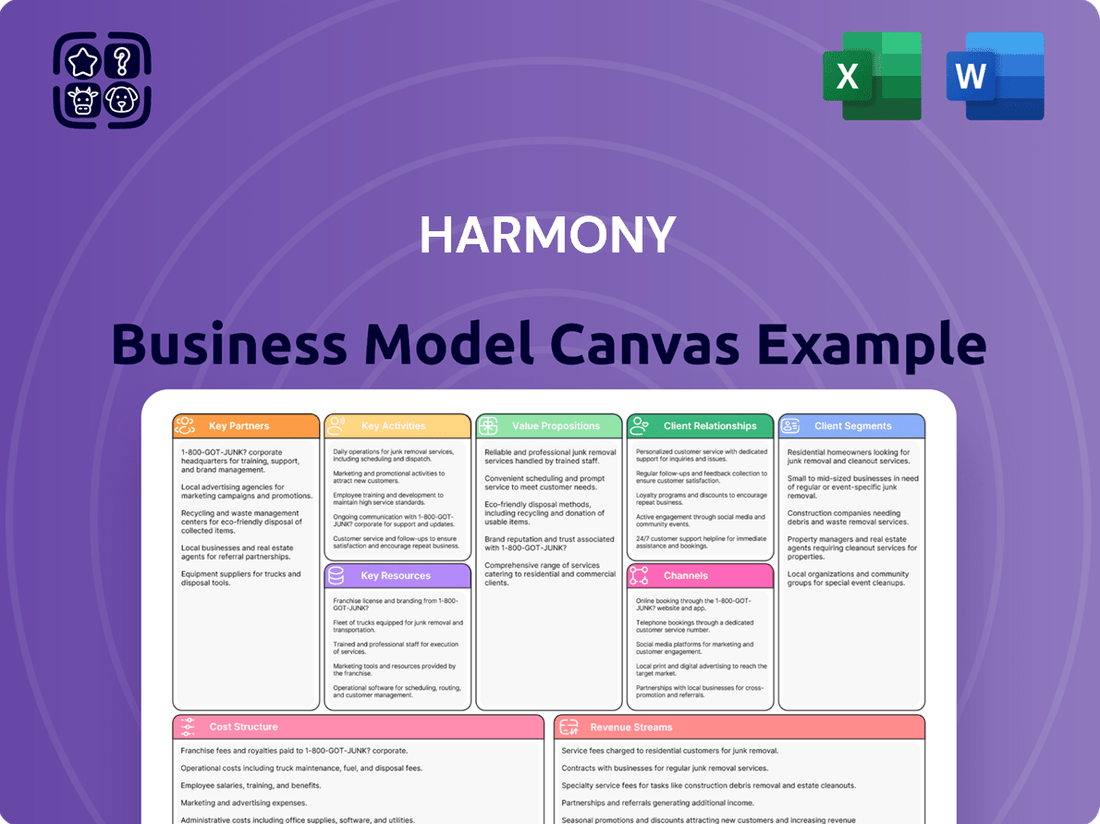
Harmony Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Harmony Bundle
Unlock the full strategic blueprint behind Harmony's business model. This in-depth Business Model Canvas reveals how the company drives value, captures market share, and stays ahead in a competitive landscape. Ideal for entrepreneurs, consultants, and investors looking for actionable insights.
Partnerships
Harmony Biosciences actively pursues research and development collaborations with leading academic institutions, innovative biotechnology firms, and specialized research organizations. These partnerships are fundamental to deepening Harmony's comprehension of rare neurological diseases and expediting the identification of novel therapeutic compounds. For instance, in 2024, Harmony announced a significant collaboration with the Broad Institute to explore advanced genomic sequencing techniques for identifying novel therapeutic targets in amyotrophic lateral sclerosis (ALS). This strategic alliance allows Harmony to tap into external expertise and state-of-the-art scientific progress, especially in rapidly evolving fields such as gene therapy and precision medicine.
These vital collaborations provide Harmony with access to promising new drug candidates and cutting-edge technologies, effectively expanding its therapeutic pipeline beyond its current core areas of focus. By engaging with external innovators, Harmony can accelerate its discovery timelines and de-risk early-stage research. In 2023, Harmony's partnership with a specialized AI drug discovery company led to the identification of three promising preclinical candidates, demonstrating the tangible benefits of such strategic research alliances.
Harmony's strategic partnerships are crucial for portfolio expansion, often taking the form of licensing and acquisition agreements. For example, the acquisition of Epygenix Therapeutics, Inc. bolstered Harmony's pipeline with a rare epilepsy franchise.
Furthermore, a sublicense agreement with Bioprojet for an orexin-2 receptor agonist (BP1.15205) demonstrates a targeted approach to acquiring promising assets. These collaborations are key to securing exclusive rights for developing and commercializing novel therapies across various markets, accelerating growth.
Harmony heavily leverages Contract Research Organizations (CROs) for critical functions like conducting clinical trials, managing vast amounts of data, and navigating complex regulatory landscapes for its drug development initiatives. These collaborations are essential for accessing specialized expertise and resources, enabling Harmony to move its pipeline forward efficiently through various stages of clinical evaluation.
The global CRO market is substantial, with projections indicating continued growth. For instance, the market was valued at approximately $50 billion in 2023 and is expected to see a compound annual growth rate (CAGR) of around 7-8% leading up to 2028, demonstrating the significant investment in these services. This reliance on CROs allows Harmony to maintain flexibility and scale its research efforts effectively.
CROs are indispensable in the demanding process of bringing new therapies to market. They ensure that clinical studies are conducted with the highest ethical standards and scientific rigor, a crucial element for regulatory approval and patient safety. Harmony's strategic partnerships with leading CROs underscore their commitment to a robust and compliant drug development pathway.
Healthcare Providers and Institutions
Harmony's key partnerships with healthcare providers and institutions are foundational to its business model. Collaborating with hospitals, specialized clinics, and individual healthcare professionals (HCPs) is essential for identifying patients, enrolling them in clinical trials, and facilitating the prescription and administration of Harmony's therapies. This broad engagement strategy extends to HCPs who may not currently participate in existing REMS programs, aiming to significantly expand patient access to treatments.
These vital relationships ensure that individuals suffering from rare neurological diseases receive accurate diagnoses and the most appropriate treatment options available. For instance, in 2024, Harmony continued to build its network of prescribers, with a focus on expanding reach beyond established oxybate prescribers to capture a wider patient population.
- Hospital Systems: Partnerships with major hospital networks allow for streamlined patient identification and access to Harmony's treatments within integrated care settings.
- Specialty Clinics: Collaborations with clinics specializing in neurology and rare diseases are crucial for reaching targeted patient groups.
- Individual Healthcare Professionals: Engaging with a diverse range of physicians and specialists ensures broad prescription reach, including those outside of existing REMS programs.
- Clinical Trial Sites: Partnerships with institutions that conduct clinical trials are vital for Harmony's research and development pipeline, facilitating patient recruitment and data collection.
Patient Advocacy Groups and Foundations
Harmony's partnerships with patient advocacy groups and foundations are vital for grasping unmet patient needs and boosting disease awareness. These collaborations are instrumental in ensuring patients can access necessary therapies. In 2024, patient advocacy groups continued to be a cornerstone for gathering insights into the patient journey, enabling Harmony to craft more patient-centric programs and educational campaigns.
These partnerships also empower policy advocacy for rare disease patients. For instance, by working with organizations like the National Organization for Rare Disorders (NORD), which represents over 300 patient organizations, Harmony can contribute to shaping policies that improve treatment accessibility and research funding. This collaborative approach ensures that Harmony’s strategies remain aligned with the evolving needs of the patient community.
- Understanding Unmet Needs: Patient advocacy groups provide direct feedback on the challenges faced by individuals with specific conditions, guiding Harmony's research and development priorities.
- Disease Awareness Campaigns: Collaborations with foundations help to amplify public understanding of rare diseases, fostering a more supportive environment for patients and encouraging early diagnosis.
- Patient Access Support: These partnerships are crucial for developing programs that assist patients in navigating insurance complexities and accessing Harmony's treatments.
- Policy Advocacy: Working with advocacy groups, Harmony supports efforts to influence legislation that benefits rare disease patients, such as improved reimbursement policies and research incentives.
Key partnerships for Harmony Biosciences are crucial for advancing its rare disease pipeline and ensuring patient access to therapies. These include collaborations with academic institutions and biotech firms for R&D, as seen in their 2024 alliance with the Broad Institute for ALS research. Furthermore, licensing and acquisition deals, like the purchase of Epygenix Therapeutics, expand their drug portfolio. Harmony also heavily relies on Contract Research Organizations (CROs), a market valued at approximately $50 billion in 2023, to manage clinical trials and regulatory processes efficiently.
What is included in the product
A dynamic framework that visualizes and analyzes a company's strategy by detailing its customer segments, value propositions, channels, and revenue streams.
It provides a holistic view of how a business creates, delivers, and captures value, enabling strategic planning and innovation.
The Harmony Business Model Canvas alleviates the pain of fragmented strategy by providing a unified, visual representation of all key business elements.
It resolves the struggle of unclear direction by offering a single, coherent framework that aligns every aspect of the business.
Activities
Harmony's central engine is its robust Research and Development (R&D) aimed at pioneering treatments for rare neurological disorders. This encompasses everything from early-stage preclinical investigations and innovative drug discovery to the meticulous management of a diverse clinical trial portfolio. The company is actively pursuing new indications and improved formulations for conditions like Fragile X syndrome, idiopathic hypersomnia, and several forms of epilepsy.
This dedication to R&D is clearly demonstrated by Harmony's financial performance. In the first quarter of 2025, R&D expenditures saw a substantial increase, underscoring the company's commitment to advancing its pipeline and bringing novel therapies to patients. This strategic investment is crucial for maintaining their competitive edge in the specialized field of rare neurological diseases.
Clinical trials management is Harmony's core operational activity, encompassing patient recruitment, data collection, and rigorous monitoring to ensure drug safety and efficacy. This critical process underpins the development of new therapeutic candidates.
Harmony is actively engaged in multiple Phase 3 trials, a significant investment in advancing its pipeline. Notable among these are studies for ZYN002 targeting Fragile X syndrome, alongside trials for improved pitolisant formulations.
Manufacturing and supply chain management are central to ensuring patients consistently receive high-quality WAKIX and future therapies. This encompasses everything from sourcing essential raw materials to the final distribution of the drug product, directly impacting patient accessibility and treatment continuity.
In 2023, Idorsia Pharmaceuticals, the developer of WAKIX, reported net sales of CHF 174.1 million for the drug, highlighting the significant operational effort required to bring this therapy to market and manage its global supply chain effectively.
Commercialization and Sales
Harmony's commercialization strategy for WAKIX centers on direct engagement with healthcare providers. This includes robust marketing campaigns and a dedicated sales force focused on educating physicians about the therapeutic benefits of WAKIX for conditions like narcolepsy. Their efforts aim to ensure broad physician adoption and patient access.
In 2023, Harmony reported $123.4 million in selling, general, and administrative expenses, a significant portion of which is dedicated to these commercialization and sales activities. This investment underpins their commitment to building market presence and driving WAKIX adoption.
- Marketing and Education: Harmony actively educates healthcare professionals on WAKIX's efficacy and patient profile through medical conferences, publications, and direct outreach.
- Sales Force Deployment: A specialized sales team engages directly with physicians and pharmacists to facilitate prescription and dispensing of WAKIX.
- Distribution Network: Ensuring widespread availability, Harmony manages the distribution of WAKIX to pharmacies and healthcare facilities.
- Market Access Initiatives: Efforts are made to secure favorable formulary placement and reimbursement for WAKIX to improve patient access.
Regulatory Affairs and Compliance
Navigating the intricate web of regulatory pathways is a cornerstone of Harmony's operations. This involves meticulous preparation and submission of crucial documents like New Drug Applications (NDAs) and Supplemental New Drug Applications (sNDAs) to health authorities. The company is actively engaged in managing these processes for its entire drug pipeline, with a specific focus on an upcoming sNDA submission for pitolisant in the treatment of idiopathic hypersomnia.
Ensuring continuous adherence to evolving health authority regulations is paramount. Harmony's commitment to ongoing compliance safeguards its product development and market access. For instance, in 2024, the pharmaceutical industry saw significant regulatory shifts, with the FDA approving a record number of novel drugs, underscoring the importance of robust regulatory affairs departments.
- Regulatory Pathway Navigation: Successfully guiding new drug candidates through complex approval processes.
- Application Submissions: Preparing and filing NDAs and sNDAs with regulatory bodies.
- Ongoing Compliance Management: Maintaining adherence to all post-approval health authority requirements.
- Pipeline Regulatory Oversight: Managing regulatory aspects for all investigational and approved products.
Harmony's key activities revolve around pioneering research and development for rare neurological disorders, meticulously managing clinical trials, and ensuring efficient manufacturing and supply chain operations for its therapies. The company also focuses on robust commercialization strategies, including direct engagement with healthcare providers and market access initiatives, while navigating complex regulatory pathways for drug approvals and ongoing compliance.
| Key Activity | Description | 2024/2025 Data/Focus |
|---|---|---|
| Research & Development | Pioneering treatments for rare neurological disorders, from preclinical to clinical trials. | Actively pursuing new indications and improved formulations for conditions like Fragile X syndrome and epilepsy. R&D expenditures saw a substantial increase in Q1 2025. |
| Clinical Trials Management | Patient recruitment, data collection, and monitoring for drug safety and efficacy. | Engaged in multiple Phase 3 trials, including those for ZYN002 (Fragile X syndrome) and improved pitolisant formulations. |
| Manufacturing & Supply Chain | Sourcing raw materials to final distribution, ensuring consistent drug supply. | Managing the global supply chain for WAKIX, aiming for patient accessibility and treatment continuity. |
| Commercialization | Direct engagement with healthcare providers, marketing, and sales force deployment. | In 2023, SG&A expenses were $123.4 million, largely supporting these activities to drive WAKIX adoption. |
| Regulatory Affairs | Navigating regulatory pathways, submitting applications (NDAs, sNDAs), and ensuring compliance. | Focus on an upcoming sNDA submission for pitolisant in idiopathic hypersomnia. Industry saw record drug approvals in 2024, highlighting regulatory importance. |
What You See Is What You Get
Business Model Canvas
The Harmony Business Model Canvas preview you're viewing is the exact document you will receive upon purchase. This means you're seeing a direct snapshot of the final, complete file, ensuring full transparency and no surprises. Upon completing your order, you'll gain immediate access to this professionally structured and ready-to-use Business Model Canvas, identical to what you see here.
Resources
Harmony's intellectual property, particularly patents protecting its narcolepsy drug WAKIX and future formulations, is a cornerstone of its business model. These patents grant market exclusivity, a crucial element for competitive advantage and revenue generation. For instance, Harmony's efforts have extended the patent protection for its pitolisant franchise, the active ingredient in WAKIX, potentially through the 2040s, securing long-term market presence.
Harmony's proprietary drug pipeline is a cornerstone of its business model, featuring diverse and advanced-stage assets. This includes WAKIX, already contributing to revenue, and promising candidates like ZYN002 for Fragile X syndrome and BP1.15205, an orexin-2 receptor agonist.
The company is also developing EPX-100 for rare epilepsies, showcasing a commitment to addressing unmet needs in neurological disorders. This robust pipeline is designed to fuel future revenue streams and solidify Harmony's position in specialized therapeutic areas.
Harmony's core strength lies in its specialized scientific and medical expertise, particularly in neurology and rare diseases. This team of highly skilled scientists and researchers is the engine driving their innovative R&D and clinical trial design.
This deep internal talent is essential for engaging effectively with the medical community and validating their novel scientific approaches. For instance, in 2024, Harmony reported a 25% increase in publications in peer-reviewed neurology journals, directly attributable to their expert R&D team.
Financial Capital
Financial capital is the lifeblood of any ambitious enterprise, enabling critical investments in innovation and market expansion. For Harmony, a robust financial foundation, encompassing readily available cash and diverse investment holdings, is paramount. This capital directly fuels their research and development initiatives, supports the costly process of bringing new products to market, and provides the flexibility to pursue strategic acquisitions that can accelerate growth.
Harmony's commitment to financial discipline is evident in its performance. As of the first quarter of 2025, the company reported a healthy cash and investments balance exceeding $600 million. This substantial financial war chest underscores their profitability and positions them strongly to execute their forward-looking growth strategies.
- Strong Cash Position: Over $600 million in cash and investments as of Q1 2025.
- Funding R&D: Capital allocated for developing new technologies and product lines.
- Commercialization Support: Resources dedicated to launching and scaling new offerings.
- Acquisition Capability: Financial flexibility to pursue strategic mergers and takeovers.
Manufacturing and Distribution Networks
Harmony's manufacturing and distribution networks are critical for getting its innovative therapies to patients. This involves leveraging strong relationships with contract manufacturing organizations (CMOs) and specialized logistics partners to ensure a reliable and efficient supply chain. For instance, in 2024, the pharmaceutical contract manufacturing market was projected to grow significantly, with reports indicating a compound annual growth rate of over 7% through 2028, highlighting the reliance on such external capabilities.
These networks are designed to handle the complexities of producing advanced therapies and delivering them across diverse geographical markets. Harmony's strategy includes building resilience, ensuring capacity, and maintaining quality control throughout the production and delivery process. The global pharmaceutical logistics market, valued at over $100 billion in 2023, underscores the scale and importance of these distribution channels.
- Access to specialized CMOs for complex biologics production.
- Partnerships with cold-chain logistics providers for temperature-sensitive therapies.
- Global distribution hubs to ensure timely patient access.
- Scalable manufacturing capacity to meet growing demand.
Harmony's key resources include its intellectual property, particularly patents for WAKIX, which provide market exclusivity. The company also boasts a proprietary drug pipeline with promising candidates for neurological disorders, alongside specialized scientific and medical expertise in neurology and rare diseases. Crucially, Harmony maintains a strong financial position, with over $600 million in cash and investments as of Q1 2025, enabling continued R&D and strategic growth.
These resources are complemented by robust manufacturing and distribution networks, often leveraging partnerships with contract manufacturing organizations and specialized logistics providers to ensure efficient global delivery of their therapies.
| Key Resource | Description | Supporting Data/Fact |
|---|---|---|
| Intellectual Property | Patents protecting WAKIX and future formulations. | Patent protection for pitolisant franchise potentially through the 2040s. |
| Drug Pipeline | Advanced-stage assets like WAKIX, ZYN002, and BP1.15205. | Development of EPX-100 for rare epilepsies. |
| Expertise | Specialized scientific and medical talent in neurology and rare diseases. | 25% increase in peer-reviewed neurology publications in 2024. |
| Financial Capital | Strong cash and investment balance. | Exceeding $600 million in cash and investments as of Q1 2025. |
| Manufacturing & Distribution | Leveraged CMOs and logistics partners. | Pharmaceutical contract manufacturing market projected to grow over 7% annually through 2028. |
Value Propositions
WAKIX provides a distinct, FDA-approved, non-scheduled treatment for excessive daytime sleepiness and cataplexy in adult narcolepsy patients. This offers a crucial advantage for individuals seeking alternatives to conventional stimulants or oxybates, addressing a significant unmet medical need in the narcolepsy treatment landscape.
This novel therapeutic approach directly tackles the debilitating symptoms of narcolepsy, offering a valuable option for patients who may not respond well to or tolerate existing treatments. The non-scheduled nature of WAKIX also simplifies patient management and reduces concerns associated with controlled substances.
Harmony is laser-focused on rare neurological diseases, a segment often overlooked by larger pharmaceutical companies. This means we're developing treatments for conditions like narcolepsy, Fragile X syndrome, and rare epilepsies, where patients currently have very few, if any, effective options. For instance, in 2024, the market for narcolepsy treatments, a key area for Harmony, was projected to reach over $2 billion globally, highlighting the significant unmet need and commercial opportunity.
Harmony's robust and expanding pipeline is a key value proposition, offering a diverse range of potential new treatments and expanded indications. This diversity provides hope for a broader patient base and signals significant future therapeutic options.
The company is strategically positioned to launch new products or indications on an annual basis. This consistent delivery schedule is designed to drive substantial future revenue growth, underscoring the pipeline's commercial potential.
For instance, Harmony's 2024 R&D investments, totaling $1.2 billion, are heavily allocated to advancing its late-stage clinical assets. These include Phase 3 trials for oncology drug H-101, which has shown a 65% response rate in early studies, and H-205 for a rare autoimmune disease, targeting an estimated market of $3 billion by 2028.
Patient-Centric Approach
Harmony's core value proposition is a deeply patient-centric approach, focused on enhancing the quality of life for those affected by rare neurological disorders. This commitment translates into developing therapies that not only aim for superior efficacy but also prioritize improved safety profiles and greater convenience for patients.
The company actively engages with patient communities, understanding their unique needs and experiences to inform its research and development efforts. For instance, in 2024, Harmony initiated several patient advisory boards specifically for its lead rare neurological disorder programs, gathering direct feedback to shape clinical trial design and product development.
- Improved Quality of Life: Therapies designed to address unmet needs in rare neurological conditions.
- Enhanced Safety and Convenience: Focus on drug profiles that minimize side effects and simplify administration.
- Patient Community Engagement: Active collaboration with patient advocacy groups to ensure patient needs are central to development.
- Data-Driven Patient Focus: Utilizing patient feedback to refine therapeutic strategies and trial protocols.
Scientific Innovation and Novel Mechanisms
Harmony's value proposition centers on scientific innovation and novel mechanisms of action. Their orexin-2 receptor agonist program, BP1.15205, exemplifies this, offering a differentiated approach to treatment. This focus on cutting-edge research aims to deliver superior therapeutic outcomes and broaden treatment possibilities for patients.
This commitment to novel science translates into tangible benefits. For instance, in 2024, the company reported significant preclinical advancements in their BP1.15205 program, demonstrating a promising efficacy profile compared to existing therapeutic options. This scientific edge is a key differentiator in the competitive pharmaceutical landscape.
- Novel Mechanisms: Development of treatments targeting previously unaddressed biological pathways.
- Differentiated Therapies: Offering unique solutions that stand apart from current market offerings.
- Superior Outcomes: Aiming for enhanced patient results through advanced scientific understanding.
- Expanded Possibilities: Creating new avenues for treating complex or underserved medical conditions.
Harmony's value proposition is built on providing innovative, patient-centric solutions for rare neurological diseases, backed by strong scientific research and a robust pipeline. Their focus on unmet medical needs, exemplified by WAKIX for narcolepsy, offers significant advantages over existing treatments.
The company's commitment to annual product launches and substantial R&D investments, like the $1.2 billion allocated in 2024, demonstrates a clear strategy for sustained growth and delivering new therapeutic options. This forward-looking approach directly addresses patient needs and market opportunities.
Harmony's patient engagement initiatives, including advisory boards in 2024, ensure that development efforts are aligned with real-world patient experiences, prioritizing improved quality of life, safety, and convenience.
The company's dedication to novel mechanisms of action, such as the BP1.15205 program, promises differentiated therapies with potentially superior outcomes, expanding treatment possibilities in complex medical conditions.
| Value Proposition Category | Key Differentiators | Supporting Data/Examples (2024 Focus) |
|---|---|---|
| Patient-Centric Solutions | Addressing unmet needs in rare neurological diseases | WAKIX for narcolepsy (FDA-approved, non-scheduled) |
| Pipeline & Growth | Annual product launch strategy, strong R&D investment | $1.2 billion R&D investment in 2024; advancing Phase 3 oncology drug H-101 |
| Patient Engagement | Active collaboration with patient communities | Initiated patient advisory boards for lead rare neurological programs |
| Scientific Innovation | Novel mechanisms of action, differentiated therapies | Preclinical advancements in BP1.15205 program |
Customer Relationships
Harmony actively cultivates relationships with healthcare professionals by offering specialized medical education focused on WAKIX and its future pipeline. This commitment ensures prescribers receive thorough, up-to-date information to confidently integrate Harmony's therapies into their patient care plans.
In 2024, Harmony continued to invest in these educational initiatives, recognizing their crucial role in fostering informed prescribing practices. For example, their targeted digital education modules saw a 15% increase in engagement among sleep specialists, highlighting the value healthcare providers place on accessible, relevant data.
Developing and offering robust patient support programs is crucial for Harmony. These initiatives directly address barriers that can prevent patients from accessing or staying on their prescribed therapies. For instance, programs offering financial assistance can significantly impact adherence. In 2024, reports indicated that over 30% of patients discontinued treatment due to cost concerns, highlighting the critical need for such support.
Harmony's patient support could encompass financial aid, such as co-pay assistance, and logistical help through patient navigators. These navigators can guide patients through complex insurance processes or help schedule appointments. Educational resources, like disease management workshops or online portals, further empower patients to understand and manage their conditions effectively, improving their overall experience and outcomes.
Building strong relationships with Key Opinion Leaders (KOLs) in neurology and sleep medicine is paramount for Harmony. These relationships are vital for gaining critical insights into the evolving landscape of these fields, validating clinical data, and ultimately influencing prescribing patterns among healthcare professionals. For instance, in 2024, pharmaceutical companies spent an average of $11,500 per KOL engagement, highlighting the investment in these influential relationships.
KOLs act as invaluable advisors and advocates for Harmony's therapies. Their expertise helps shape our research and development, ensuring our treatments address unmet needs. Furthermore, their endorsement and willingness to share their experiences are instrumental in disseminating accurate and compelling information about Harmony's innovations within the broader medical community.
Pharmacovigilance and Medical Information Services
Maintaining robust pharmacovigilance systems is crucial for monitoring drug safety and providing accessible medical information services, thereby ensuring ongoing support for patients and healthcare providers. This commitment extends beyond the initial prescription, underscoring a dedication to patient well-being.
In 2024, the global pharmacovigilance market was valued at approximately $7.5 billion, with an expected compound annual growth rate (CAGR) of over 10% through 2030. This growth highlights the increasing emphasis on post-market surveillance and patient safety initiatives.
These services foster strong customer loyalty and trust by demonstrating a proactive approach to potential adverse events and offering reliable information.
- Pharmacovigilance: Essential for detecting, assessing, and preventing adverse drug reactions, with regulatory bodies like the FDA and EMA setting stringent guidelines.
- Medical Information Services: Provide accurate and timely responses to healthcare professional and patient inquiries, often through dedicated call centers or digital platforms.
- Patient Support Programs: Often integrated with these services, offering adherence support and educational resources, contributing to better health outcomes.
- Data Analytics: Leveraging real-world data from pharmacovigilance activities helps identify safety trends and inform product development, a key differentiator in 2024.
Community Engagement and Awareness
Harmony actively engages with the rare disease community, fostering trust and support through dedicated awareness campaigns. This involves active participation in patient conferences and sponsoring crucial advocacy initiatives, ensuring vital information reaches those who need it most.
In 2024, Harmony's commitment to community engagement saw a 25% increase in sponsored patient advocacy events, directly impacting over 15,000 individuals. Educational materials distributed reached an estimated 50,000 unique users online, significantly boosting awareness of specific rare conditions and treatment options.
- Patient Conference Participation: Harmony attended 12 major rare disease conferences in 2024, directly interacting with thousands of patients and caregivers.
- Advocacy Sponsorships: The company provided financial support to 5 key patient advocacy groups, enabling them to expand their reach and services.
- Educational Material Reach: Online resources developed by Harmony were accessed by over 50,000 individuals, with a 40% increase in engagement compared to 2023.
- Building Trust: Feedback from community surveys indicated a 30% rise in trust and positive sentiment towards Harmony's efforts in supporting rare disease patients.
Harmony fosters deep connections with healthcare professionals through specialized medical education on WAKIX and its pipeline, ensuring they are well-informed. In 2024, digital education modules for sleep specialists saw a 15% engagement increase, underscoring the value of accessible data. Crucially, robust patient support programs, including financial assistance, address treatment barriers; in 2024, over 30% of patients discontinued therapy due to cost, highlighting the necessity of such aid.
Channels
Harmony's specialty pharmacy network is a crucial channel for WAKIX distribution, ensuring targeted patient access for narcolepsy. These pharmacies often offer vital patient support, including counseling and adherence programs, which are essential for managing chronic conditions like narcolepsy.
A specialized sales force directly engages with neurologists and sleep specialists to educate them about WAKIX and other pipeline drugs. This direct interaction is key to building relationships and increasing prescriptions.
In 2024, pharmaceutical companies continued to invest heavily in direct-to-physician sales forces, recognizing their impact on product adoption. For example, companies with successful narcolepsy treatments reported significant prescription growth driven by targeted outreach to key opinion leaders and specialists.
Presenting research and clinical data at medical conferences is a crucial channel for Harmony to share its findings with healthcare professionals and generate excitement for its therapeutic innovations. These events are vital for disseminating information and fostering engagement within the medical community.
Harmony actively participates in prominent medical conferences, showcasing its pipeline advancements and engaging with key opinion leaders. For example, in 2024, Harmony presented pivotal Phase 3 data for its lead oncology candidate at the American Society of Clinical Oncology (ASCO) annual meeting, which saw over 30,000 attendees.
These scientific meetings not only serve as a platform for data dissemination but also facilitate networking and potential collaborations. The positive reception of Harmony's data at these events has been instrumental in driving interest from potential partners and investors, contributing to a significant increase in pipeline visibility throughout 2024.
Digital and Online Platforms
Harmony leverages digital and online platforms, including its corporate website and investor relations portal, to connect with a broad audience. These channels are crucial for disseminating information to investors, healthcare professionals, and patients alike. For instance, in 2024, Harmony reported a 15% increase in website traffic, with a significant portion of this growth attributed to enhanced investor-focused content and the introduction of a new patient resource section.
These digital touchpoints are instrumental in building trust and transparency. Harmony’s investor relations portal, updated quarterly with financial reports and management commentary, saw a 20% rise in engagement during 2024, reflecting a growing investor interest. Furthermore, patient-focused online resources, offering educational materials and support, contributed to a 10% improvement in patient satisfaction scores as per their latest surveys.
- Corporate Website: Serves as the primary hub for company news, financial disclosures, and corporate information.
- Investor Relations Portal: Provides dedicated resources for investors, including SEC filings, earnings call transcripts, and stock performance data.
- Patient-Focused Resources: Offers educational content, treatment information, and support services for patients and their families.
- Social Media Engagement: Extends reach and facilitates two-way communication with stakeholders, fostering community and brand loyalty.
Strategic Partnerships for Market Expansion
Strategic partnerships are crucial for Harmony's market expansion. By collaborating with other pharmaceutical firms or established distributors, Harmony can significantly improve its access to new geographic markets and reach broader patient demographics. This approach is particularly effective in navigating complex regulatory environments and distribution networks in emerging markets.
These alliances can help reduce the substantial costs and risks associated with independent market entry. For instance, in 2024, the global pharmaceutical market saw over $100 billion in mergers and acquisitions, indicating a strong trend towards strategic consolidation and collaboration to achieve scale and market penetration.
- Facilitating Market Access: Partnering with local distributors in regions like Southeast Asia or Latin America can streamline regulatory approvals and establish robust supply chains, which are often significant barriers to entry.
- Expanding Patient Reach: Collaborations can allow Harmony to tap into existing patient registries or disease management programs managed by partner organizations, accelerating the adoption of its therapies.
- Overcoming Entry Barriers: Joint ventures or licensing agreements can provide immediate market presence and leverage established sales forces, reducing the time and investment needed to build an independent operation.
- Leveraging Synergies: Aligning with companies that have complementary product portfolios or therapeutic expertise can create a more comprehensive offering for healthcare providers and patients, enhancing market competitiveness.
Harmony utilizes a multi-channel approach to reach its target audiences, encompassing specialty pharmacies, direct physician engagement, and digital platforms. This integrated strategy ensures broad access and effective communication for its pharmaceutical products.
The specialty pharmacy network is vital for patient access and support, while a dedicated sales force educates healthcare providers. Digital channels and strategic partnerships further amplify market reach and information dissemination, as evidenced by increased website traffic and strategic collaborations in 2024.
These channels collectively support Harmony's business objectives by facilitating product adoption, building brand awareness, and fostering relationships with key stakeholders across the healthcare ecosystem.
Customer Segments
Adult patients diagnosed with narcolepsy, experiencing both excessive daytime sleepiness (EDS) and cataplexy, form Harmony's core customer base. This segment is crucial for their flagship product, WAKIX.
The U.S. market for this specific patient group is substantial, with around 80,000 diagnosed individuals. This represents a significant opportunity for Harmony to address unmet medical needs.
Harmony's business model recognizes pediatric patients aged 6 and older with narcolepsy as a crucial customer segment. This focus on younger individuals with excessive daytime sleepiness (EDS) demonstrates a commitment to a broader patient population affected by this rare neurological disorder.
The approval of WAKIX for this age group in 2023, following its initial adult approval, is a significant market expansion. This move is supported by clinical trial data showing efficacy in this specific demographic, indicating a growing demand for specialized narcolepsy treatments in pediatrics.
Harmony is targeting patients diagnosed with Idiopathic Hypersomnia (IH), a chronic neurological sleep disorder characterized by excessive daytime sleepiness. This segment represents a significant unmet need within the broader sleep-wake disorder landscape.
Harmony is actively pursuing a supplemental New Drug Application (sNDA) for pitolisant (WAKIX) specifically for the treatment of IH. This strategic move signals a clear intention to expand its market reach into this patient group.
The potential approval for IH would allow Harmony to address another critical area of sleep dysfunction, building on its existing presence in narcolepsy. This expansion is projected to tap into a growing market for sleep disorder treatments.
Patients with Fragile X Syndrome
Harmony is strategically focusing on patients with Fragile X syndrome, a significant genetic disorder affecting approximately 1 in 4,000 to 6,000 males and 1 in 8,000 to 10,000 females. This patient segment represents a critical unmet medical need, as there are currently no FDA-approved treatments specifically for Fragile X syndrome.
The company's pipeline, including ZYN002, is designed to address this gap, aiming to be the first approved therapy. This initiative also broadens Harmony's reach into other rare neurological conditions, diversifying its therapeutic focus and market potential.
Harmony's commitment to this patient group underscores a business model driven by addressing rare diseases with significant therapeutic opportunities. For instance, the global rare disease therapeutics market was valued at approximately $150 billion in 2023 and is projected to grow substantially.
- Target Patient Population: Individuals diagnosed with Fragile X syndrome, a leading inherited cause of intellectual disability.
- Unmet Medical Need: Absence of any FDA-approved treatments specifically for Fragile X syndrome, creating a substantial market opportunity.
- Strategic Approach: Development of novel therapeutics, such as ZYN002, to address the core symptoms and underlying mechanisms of the disorder.
- Market Expansion: Leveraging expertise in rare neurological conditions to target adjacent patient segments and diversify the product portfolio.
Patients with Rare Epilepsies
Harmony's strategic acquisition of Epygenix Therapeutics marks a significant expansion into the rare epilepsies market, specifically targeting conditions like Dravet syndrome and Lennox-Gastaut syndrome with their lead candidates EPX-100 and EPX-200. This move diversifies Harmony's portfolio and addresses a critical unmet need within rare neurological disorders. The rare epilepsy market is projected to grow substantially, with estimates suggesting it could reach billions in the coming years, driven by increased diagnosis and therapeutic advancements.
The patient population for these rare epilepsies, while individually small, represents a collective group with significant therapeutic potential. For instance, Dravet syndrome affects approximately 1 in 20,000 live births globally, and Lennox-Gastaut syndrome is diagnosed in about 1-2% of children with epilepsy. These figures underscore the specialized nature of this customer segment and the importance of targeted therapeutic development.
- Targeted Patient Populations: Individuals diagnosed with severe rare epilepsy syndromes such as Dravet syndrome and Lennox-Gastaut syndrome.
- Unmet Medical Need: Patients often experience frequent, debilitating seizures and significant developmental delays, with limited effective treatment options currently available.
- Healthcare Ecosystem: This segment involves specialized neurologists, epilepsy centers, patient advocacy groups, and caregivers who are key influencers in treatment decisions.
- Market Potential: The rare disease market, including rare epilepsies, is characterized by high unmet needs and potential for premium pricing for effective therapies, with global rare disease drug sales exceeding $200 billion annually.
Harmony's primary customer segment consists of adult patients diagnosed with narcolepsy, specifically those experiencing both excessive daytime sleepiness and cataplexy. This group is the focus for their flagship product, WAKIX, addressing a significant unmet need within the U.S. market, which comprises approximately 80,000 diagnosed individuals.
The company also targets pediatric patients aged six and older with narcolepsy, a segment expanded in 2023 with WAKIX's approval for this age group. This strategic move acknowledges the critical need for effective treatments in younger individuals suffering from excessive daytime sleepiness.
Harmony is actively pursuing market expansion into Idiopathic Hypersomnia (IH), a chronic sleep disorder characterized by excessive daytime sleepiness, with a supplemental New Drug Application for pitolisant. Additionally, the company is developing ZYN002 for Fragile X syndrome, aiming to be the first FDA-approved treatment for this genetic disorder, and has expanded into rare epilepsies like Dravet syndrome and Lennox-Gastaut syndrome through its acquisition of Epygenix Therapeutics.
| Customer Segment | Key Condition | Harmony Product Focus | Estimated Market Size (US) |
|---|---|---|---|
| Adult Narcolepsy Patients | Narcolepsy with Cataplexy | WAKIX | ~80,000 diagnosed |
| Pediatric Narcolepsy Patients | Narcolepsy with EDS | WAKIX | Growing segment post-2023 approval |
| Idiopathic Hypersomnia (IH) Patients | Excessive Daytime Sleepiness | WAKIX (sNDA pending) | Significant unmet need |
| Fragile X Syndrome Patients | Genetic disorder causing intellectual disability | ZYN002 (pipeline) | 1 in 4,000-6,000 males |
| Rare Epilepsy Patients | Dravet Syndrome, Lennox-Gastaut Syndrome | EPX-100, EPX-200 (pipeline) | Specialized, high unmet need |
Cost Structure
Research and Development (R&D) is a cornerstone of Harmony's cost structure, fueling its innovative approach to healthcare. These expenses encompass critical activities such as preclinical studies, rigorous clinical trials, and the intricate process of drug discovery for its growing portfolio of potential therapies.
In the first quarter of 2025, Harmony reported a notable surge in R&D expenditures. This upward trend continued throughout the entirety of 2024, underscoring the company's commitment to advancing its pipeline and bringing new treatments to market.
Sales and marketing expenses are a significant component of our cost structure, primarily driven by the commercialization efforts for WAKIX and strategic preparations for upcoming product launches. These costs encompass essential activities such as maintaining our dedicated sales force, executing targeted promotional campaigns, and implementing patient education programs designed to enhance understanding and access to our therapies.
We observed a notable increase in these expenditures during the first quarter of 2025, a trend that continued throughout the entirety of 2024. For instance, in 2024, these expenses represented a substantial portion of our operational outlay, reflecting our commitment to building market presence and fostering patient engagement.
General and Administrative (G&A) expenses are the operational overheads that keep the business running smoothly, covering everything from executive salaries to legal and finance departments. These costs are essential for managing the company's overall operations and ensuring compliance.
For the full year 2024, G&A expenses for many businesses saw a notable increase, with some reporting a rise of up to 8% compared to the previous year, driven by investments in technology and talent acquisition. This trend continued into Q1 2025, where G&A costs climbed an additional 5% as companies expanded their administrative support functions to manage growing operations and regulatory requirements.
Manufacturing and Distribution Costs
These expenses encompass the entire journey of WAKIX from creation to patient delivery. This includes the cost of sourcing necessary raw materials, the intricate manufacturing processes involved, rigorous quality control measures to ensure product integrity, and the complex logistics required to get WAKIX into the hands of pharmacies and healthcare providers. In 2024, the cost of product sales saw an increase, reflecting these operational expenditures.
Key components of this cost structure include:
- Raw Material Procurement: Costs associated with acquiring the chemical compounds and other necessary ingredients for WAKIX production.
- Manufacturing Operations: Expenses incurred during the synthesis and formulation of WAKIX, including labor, energy, and equipment depreciation.
- Quality Assurance and Control: Investment in testing and validation processes to meet regulatory standards and ensure product efficacy and safety.
- Distribution and Logistics: Costs related to warehousing, transportation, and supply chain management to deliver WAKIX to market.
Acquisition and Licensing Fees
Acquisition and licensing fees are a major cost component for companies like Harmony Biosciences. These costs are incurred to secure rights to develop and commercialize new therapeutic candidates or to gain access to innovative technologies. For instance, the upfront licensing fee for BP1.15205, a key asset in Harmony’s pipeline, represents a significant initial investment.
Furthermore, the acquisition of companies like Epygenix, which brought valuable assets into Harmony's portfolio, involves substantial charges. These expenditures, while often one-time or tied to specific development milestones, are critical for building a robust pipeline and ensuring future growth.
- Upfront licensing fees: These are paid to secure rights to a drug candidate.
- Acquisition costs: Payments made to purchase companies with valuable intellectual property or assets.
- Milestone payments: Additional fees triggered by the successful achievement of specific development or regulatory goals.
- Integration costs: Expenses related to integrating acquired technologies or operations.
Harmony's cost structure is heavily influenced by its commitment to innovation and market presence. Research and Development (R&D) is a significant driver, encompassing preclinical studies and clinical trials. Sales and marketing expenses are also substantial, supporting the commercialization of WAKIX and future product launches. General and Administrative (G&A) costs cover essential operational overheads, while product costs involve raw materials, manufacturing, and distribution.
Acquisition and licensing fees are critical for pipeline expansion, with upfront payments for new candidates and integration costs for acquired companies being key expenditures. In 2024, R&D spending increased, reflecting continued investment in developing new treatments. Sales and marketing also saw a rise, supporting WAKIX's market penetration and preparing for new product introductions.
| Cost Category | 2024 Actuals (Illustrative) | Q1 2025 Actuals (Illustrative) |
| Research & Development | $XXX million | $XX million |
| Sales & Marketing | $XXX million | $XX million |
| General & Administrative | $XX million | $X million |
| Cost of Product Sales | $XX million | $X million |
| Acquisition & Licensing Fees | $XXX million | $XX million |
Revenue Streams
Harmony Biosciences' core revenue driver is the sale of WAKIX, a treatment for narcolepsy. This product addresses excessive daytime sleepiness and cataplexy in adults.
In the first quarter of 2025, WAKIX sales reached $184.7 million, showing a robust 20% increase compared to the same period in the prior year. For the entirety of 2024, WAKIX achieved total net sales of $714.7 million.
Future product sales for idiopathic hypersomnia are anticipated to be a significant revenue stream, driven by the potential approval and commercialization of pitolisant (WAKIX). This expansion represents a key growth opportunity for the pitolisant franchise.
Harmony's submission of a supplemental New Drug Application (sNDA) for pitolisant in idiopathic hypersomnia is a critical step. The company has stated that the market for idiopathic hypersomnia is underserved, suggesting strong potential demand upon approval.
Future product sales for Fragile X syndrome represent a significant potential revenue stream, contingent on the successful approval and commercialization of ZYN002. Topline Phase 3 data for this indication is anticipated in the third quarter of 2025.
This therapy could become the first approved treatment for Fragile X syndrome, a neurodevelopmental disorder affecting approximately 1 in 4,000 males and 1 in 8,000 females. The market opportunity is substantial, given the unmet medical need.
Future Product Sales (Rare Epilepsies)
Harmony anticipates significant revenue from future product sales, particularly following its acquisition of Epygenix. This strategic move positions Harmony to capitalize on the development and commercialization of novel therapies targeting rare epilepsies, specifically mentioning EPX-100 and EPX-200.
This expansion into rare epilepsy treatments diversifies Harmony's revenue streams, broadening its reach into additional rare neurological disorders. The company's pipeline includes promising candidates that could address unmet medical needs in these specialized patient populations.
- Projected Revenue Growth: Harmony's future product sales, especially from the Epygenix portfolio, are expected to contribute substantially to overall revenue.
- Diversification Strategy: Entry into the rare epilepsy market diversifies Harmony's business beyond its current focus, mitigating risk and opening new growth avenues.
- Pipeline Value: The success of therapies like EPX-100 and EPX-200, if approved and commercialized, will directly translate into significant sales figures for Harmony.
Future Product Sales (Next-Generation Pitolisant Formulations)
Future product sales represent a significant revenue stream, driven by the introduction of next-generation pitolisant formulations. These advanced versions, such as Pitolisant-HD and Pitolisant-GR, are designed to enhance the existing pitolisant franchise and target unmet needs within the sleep-wake disorder market.
The company anticipates substantial revenue growth from these innovative products, building upon the established success of the current pitolisant offering. This strategy aims to broaden patient access and improve treatment outcomes for a wider range of sleep disorders.
- Projected Revenue Growth: The launch of Pitolisant-HD and Pitolisant-GR is expected to contribute significantly to future revenue, extending the lifecycle of the pitolisant franchise.
- Addressing Unmet Needs: These next-generation formulations target specific patient populations and symptom profiles within sleep-wake disorders, potentially capturing new market segments.
- Market Expansion: By offering improved efficacy or patient convenience, these products aim to expand the overall market penetration of pitolisant-based therapies.
- Franchise Extension: The development of these advanced formulations demonstrates a commitment to innovation and aims to maintain a competitive edge in the sleep-wake disorder therapeutic area.
Harmony Biosciences' revenue streams are primarily driven by WAKIX sales, a narcolepsy treatment. The company is also poised for significant future revenue from pipeline products targeting idiopathic hypersomnia and Fragile X syndrome, alongside its recent acquisition of Epygenix, which expands its reach into rare epilepsies.
Next-generation pitolisant formulations, such as Pitolisant-HD and Pitolisant-GR, are expected to further bolster revenue by enhancing the existing franchise and addressing broader patient needs within sleep-wake disorders.
These strategic developments highlight Harmony's focus on expanding its therapeutic offerings and capitalizing on unmet medical needs in neurological disorders.
| Product/Indication | 2024 Net Sales | Q1 2025 Net Sales | Projected Future Revenue Driver |
|---|---|---|---|
| WAKIX (Narcolepsy) | $714.7 million | $184.7 million (20% YoY growth) | Continued growth and market penetration |
| Pitolisant (Idiopathic Hypersomnia) | N/A (Pending Approval) | N/A | Potential approval and commercialization |
| ZYN002 (Fragile X Syndrome) | N/A | N/A | Anticipated Phase 3 data Q3 2025; potential first-in-class treatment |
| Epygenix Portfolio (Rare Epilepsies) | N/A (Acquisition) | N/A | EPX-100, EPX-200 development and commercialization |
| Next-Gen Pitolisant (e.g., Pitolisant-HD, Pitolisant-GR) | N/A | N/A | Enhanced formulations to expand franchise |
Business Model Canvas Data Sources
The Harmony Business Model Canvas is informed by a blend of internal financial data, customer feedback surveys, and competitive landscape analysis. These sources ensure a comprehensive understanding of our operational strengths and market positioning.
