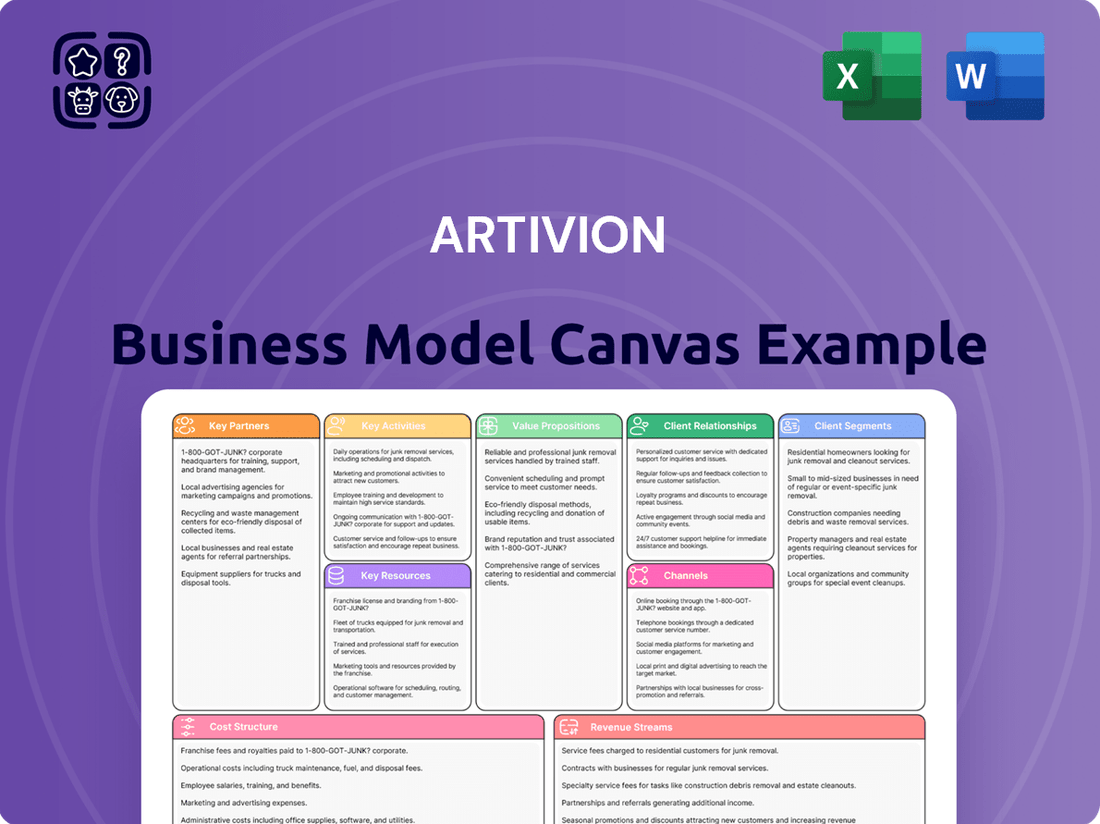
Artivion Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Artivion Bundle
Unlock the strategic core of Artivion's success with our comprehensive Business Model Canvas. Discover how they create and deliver value, manage key resources, and build strong customer relationships. This detailed analysis is your key to understanding their competitive edge.
Dive into the operational blueprint of Artivion with our full Business Model Canvas. It meticulously outlines their customer segments, revenue streams, and cost structure, offering invaluable insights for strategic planning and competitive analysis. Get the complete picture today.
Partnerships
Artivion collaborates with hospitals and surgical centers worldwide, acting as a vital channel for distributing its advanced medical devices and implantable human tissues essential for cardiac and vascular surgeries. These relationships are fundamental for ensuring broad product availability and facilitating the integration of Artivion's innovative solutions directly into established patient care protocols.
These strategic alliances are critical for Artivion's business model, enabling not only efficient product delivery but also providing essential platforms for surgeon education and hands-on training with their specialized aortic, cardiac, and vascular repair technologies. For instance, in 2024, Artivion continued to expand its training programs, reaching over 5,000 surgeons across key international markets.
Artivion's success hinges on deep collaboration with cardiac and vascular surgeons. These professionals are not just users of Artivion's products but also crucial partners in innovation, providing essential insights for product development.
This partnership extends to critical clinical trials, such as the PERSEVERE study for their Advanced Medical Device System (AMDS). Surgeons' participation in these trials is vital for gathering robust data on product efficacy and safety, directly informing Artivion's research and development pipeline.
Furthermore, surgeons contribute significantly through post-market studies, offering real-world feedback that helps Artivion refine existing products and identify opportunities for new solutions. This continuous feedback loop ensures Artivion's offerings remain at the forefront of medical technology.
Artivion actively collaborates with research and development institutions to push the boundaries of medical innovation, particularly in areas like cardiac and vascular surgery. These partnerships are crucial for developing next-generation implantable devices and tissue-based solutions, ensuring the company remains at the forefront of its field.
For example, collaborations with universities and research centers facilitate the rigorous testing and validation of new products, such as advancements related to their On-X Aortic Valve or the AMDS Hybrid Prosthesis. These academic ties are instrumental in conducting vital preclinical studies and clinical trials, which are essential for regulatory approval and market adoption.
These strategic alliances help Artivion to access cutting-edge scientific knowledge and specialized expertise, accelerating the translation of novel concepts into tangible medical advancements. Such research partnerships are key to exploring new therapeutic applications and enhancing the efficacy of their existing product portfolio.
Regulatory Bodies (e.g., FDA)
Maintaining robust relationships and ensuring compliance with regulatory bodies such as the U.S. Food and Drug Administration (FDA) is a cornerstone of Artivion's business model. These partnerships are essential for navigating the complex approval processes required for medical devices.
Timely approvals from entities like the FDA are crucial for introducing new devices and expanding indications for existing ones. For instance, the Humanitarian Device Exemption (HDE) for the AMDS Hybrid Prosthesis highlights the significance of these regulatory collaborations for market access and successful commercialization.
- Regulatory Approvals: Securing FDA clearance is paramount for product launches and market entry.
- Compliance and Safety: Adhering to strict regulatory standards ensures patient safety and product integrity.
- Market Access: Regulatory approvals, like the HDE for AMDS, unlock commercial opportunities and patient access to innovative treatments.
- Product Lifecycle Management: Ongoing engagement with regulatory bodies supports post-market surveillance and potential label expansions.
Suppliers of Raw Materials and Components
Artivion relies heavily on a network of trusted suppliers for the critical raw materials and components needed to produce its advanced medical devices. These partnerships are fundamental to maintaining the high quality and consistent availability of specialized inputs. For instance, securing a reliable supply of bovine blood protein is paramount for the production of their BioGlue surgical sealant, a key product in their portfolio.
Furthermore, the company depends on suppliers for essential components used in the manufacturing of its stent grafts and heart valves. Ensuring the stability and integrity of this supply chain is not just about material acquisition; it directly impacts Artivion's ability to maintain uninterrupted production schedules and meet global demand for its life-saving technologies.
In 2023, Artivion reported total cost of goods sold of $323.5 million, highlighting the significant investment in its supply chain. The company’s focus on supplier relationships is therefore a strategic imperative for operational continuity and product innovation.
- Bovine Blood Protein: Essential for BioGlue, requiring specialized sourcing and quality control.
- Component Sourcing: Critical for stent grafts and heart valves, demanding precision and reliability.
- Supply Chain Resilience: Key to preventing production disruptions and ensuring market availability.
- Supplier Quality Management: Underpins the safety and efficacy of Artivion's medical products.
Artivion's key partnerships are vital for its global operations and innovation. These include collaborations with hospitals and surgical centers for product distribution and surgeon training, research institutions for R&D, and regulatory bodies like the FDA for market access.
The company also relies on a robust network of suppliers for critical raw materials, such as bovine blood protein for BioGlue, ensuring the quality and availability of its medical devices.
These strategic alliances directly support Artivion's ability to bring life-saving cardiac and vascular solutions to patients worldwide, with significant investment in supplier relationships to maintain operational continuity.
| Partner Type | Role | Example | Impact |
| Hospitals & Surgical Centers | Distribution, Training | Global surgical sites | Product availability, Surgeon proficiency |
| Surgeons | Product Use, Innovation Input | Cardiac & Vascular Specialists | Product development, Clinical trial data |
| Research Institutions | R&D, Validation | Universities, Research Centers | Next-generation product development |
| Regulatory Bodies | Approval, Compliance | FDA | Market access, Patient safety |
| Suppliers | Raw Materials, Components | Bovine blood protein suppliers | Product quality, Production continuity |
What is included in the product
A detailed framework outlining Artivion's approach to delivering life-saving cardiac and vascular solutions, focusing on key patient and surgeon segments, specialized distribution channels, and innovative product value propositions.
This model details Artivion's revenue streams from implantable device sales and partnerships, alongside its cost structure driven by R&D and manufacturing, all designed to support its mission of improving patient outcomes.
Artivion's Business Model Canvas serves as a pain point reliever by providing a clear, visual roadmap of their complex business, simplifying the understanding of how they deliver life-saving medical devices.
It acts as a pain point reliever by condensing Artivion's intricate operations into a single, digestible page, making it easier to identify and address potential inefficiencies or strategic gaps.
Activities
Artivion's core activity is deeply rooted in research and development, focusing on pioneering medical devices and implantable human tissues for cardiac and vascular surgeries. This commitment drives their innovation in a highly specialized field.
A significant part of this R&D involves conducting crucial clinical trials. For instance, the PERSEVERE study is evaluating the AMDS Hybrid Prosthesis, a key initiative to demonstrate the efficacy and safety of their advanced products. This ongoing research is vital for market adoption and patient outcomes.
Furthermore, Artivion actively pursues regulatory approvals for both new product launches and expanded indications for existing devices. In 2023, the company achieved several regulatory milestones, including FDA approval for the On-X Ascending Aortic Conduit, underscoring their progress in bringing innovative solutions to market.
Artivion's core manufacturing and processing activities involve creating life-saving medical devices. This includes producing aortic stent grafts, crucial for treating aortic aneurysms, and surgical sealants that help control bleeding during operations. They also manufacture the advanced On-X mechanical heart valves, a significant innovation in cardiac surgery.
Beyond device manufacturing, Artivion processes human tissues for cardiac and vascular implants. This intricate work requires highly specialized facilities and rigorous adherence to stringent quality control measures. For instance, in 2023, Artivion reported revenue of $759.7 million, reflecting the significant demand and scale of their operations in these critical healthcare sectors.
Artivion's sales and marketing efforts are geared towards promoting its extensive range of medical devices for aortic, cardiac, and vascular repair. These activities target healthcare providers across more than 100 countries, ensuring a global reach for their innovative solutions.
The company employs direct sales teams who engage with cardiac and vascular surgeons, building relationships and educating them on Artivion's product benefits. This personal approach is crucial for introducing complex medical technologies.
A significant part of their strategy involves participating in major medical conferences. For example, in 2024, Artivion actively showcased its offerings at key industry events, facilitating direct interaction with potential customers and thought leaders in cardiovascular surgery.
Developing targeted marketing campaigns further amplifies their message. These strategies aim to highlight the clinical advantages and patient outcomes associated with Artivion's repair solutions, driving adoption among surgical professionals.
Regulatory Affairs and Compliance
Artivion's key activities heavily involve managing regulatory affairs and ensuring compliance across diverse global markets. This is crucial for obtaining and sustaining approvals for their innovative medical devices and biological tissues. The company must meticulously prepare and submit applications to regulatory bodies such as the U.S. Food and Drug Administration (FDA) and similar international agencies.
Maintaining ongoing adherence to all applicable medical device regulations is paramount. This includes post-market surveillance, quality management system updates, and responding to any regulatory inquiries or changes. For instance, in 2024, companies in the medical device sector faced increased scrutiny on cybersecurity and data privacy related to connected devices, a trend Artivion would actively address.
- Navigating Global Regulatory Landscapes: Securing and maintaining approvals for medical devices and tissues in regions like the U.S., Europe, and Asia.
- FDA Submissions and Compliance: Preparing and filing applications with the FDA for new products and ensuring ongoing adherence to FDA regulations.
- International Regulatory Adherence: Complying with the specific medical device regulations of various countries where products are marketed.
Clinical Education and Training
Artivion's commitment to clinical education and training is a cornerstone of its business model. They actively engage in educating surgeons and healthcare professionals on the effective and safe utilization of their advanced medical devices and implantable tissues. This focus on knowledge transfer is crucial for maximizing patient benefits and encouraging the integration of Artivion's innovative offerings into standard surgical procedures.
This educational outreach directly supports the adoption of their technologies. For instance, Artivion offers specialized training programs designed to familiarize users with the nuances of their On-X Aortic Valve, a key product. Such programs are vital for building surgeon confidence and ensuring the best possible patient outcomes, thereby driving market penetration and reinforcing Artivion's reputation for quality and support.
- Surgeon Training Programs: Artivion conducts hands-on workshops and simulation sessions for surgeons.
- Product Adoption Support: Education facilitates the wider use of Artivion's implantable tissues and devices.
- Patient Outcome Focus: Training emphasizes techniques that lead to improved patient recovery and long-term results.
Artivion's key activities are centered on the innovation, manufacturing, and global distribution of specialized medical devices for cardiac and vascular repair. This includes extensive research and development to create pioneering implantable human tissues and advanced prosthetics, supported by rigorous clinical trials like the PERSEVERE study to demonstrate product efficacy. The company also focuses on securing regulatory approvals, as seen with the 2023 FDA approval for the On-X Ascending Aortic Conduit, and maintains robust manufacturing processes for products such as aortic stent grafts and mechanical heart valves.
Sales and marketing efforts are crucial, targeting healthcare providers in over 100 countries through direct sales teams and participation in major industry events, including prominent medical conferences in 2024. Artivion also prioritizes clinical education and training for surgeons, offering specialized programs to ensure the safe and effective use of their technologies, thereby fostering product adoption and improving patient outcomes.
The company's operations are underpinned by meticulous management of regulatory affairs and compliance across international markets, involving continuous adherence to medical device regulations and proactive engagement with bodies like the FDA. This comprehensive approach ensures market access and sustained product availability.
| Key Activity Area | Description | Example/Data Point |
|---|---|---|
| Research & Development | Pioneering medical devices and implantable human tissues for cardiac and vascular surgeries. | PERSEVERE study evaluating the AMDS Hybrid Prosthesis. |
| Manufacturing & Processing | Producing life-saving medical devices and processing human tissues. | Manufactures aortic stent grafts, surgical sealants, and On-X mechanical heart valves. |
| Sales & Marketing | Promoting medical devices to healthcare providers globally. | Reached healthcare providers in over 100 countries; active participation in 2024 medical conferences. |
| Regulatory Affairs & Compliance | Ensuring adherence to global medical device regulations. | Achieved FDA approval for On-X Ascending Aortic Conduit in 2023. |
| Clinical Education & Training | Educating healthcare professionals on device utilization. | Offers specialized training programs for products like the On-X Aortic Valve. |
Preview Before You Purchase
Business Model Canvas
The Artivion Business Model Canvas preview you are viewing is the actual document you will receive upon purchase. This means you're seeing the complete, unedited file, ensuring transparency and no hidden surprises. Once your order is processed, you'll gain full access to this exact Business Model Canvas, ready for immediate use and customization.
Resources
Artivion's intellectual property, particularly its patents for groundbreaking medical devices such as the On-X mechanical heart valves and the AMDS Hybrid Prosthesis, forms a cornerstone of its business model. These patents safeguard proprietary technologies, ensuring a distinct competitive edge within the specialized cardiac and vascular surgery sector.
In 2023, Artivion's commitment to innovation was evident, with significant investment in research and development to expand its patent portfolio. This strategic focus on protecting its technological advancements is vital for maintaining market leadership and driving future growth.
Artivion's specialized manufacturing facilities are crucial to its business model, enabling the high-quality production of its medical devices and the careful processing of human tissues. These facilities are strategically located in Atlanta, Georgia; Austin, Texas; and Hechingen, Germany, ensuring both operational efficiency and access to key markets.
These sites are outfitted with advanced infrastructure and cutting-edge technology, essential for meeting the stringent demands of medical device manufacturing and tissue processing. For instance, their tissue processing operations adhere to rigorous regulatory standards, a critical factor in the medical field.
In 2023, Artivion reported significant investments in its manufacturing capabilities, underscoring the importance of these specialized facilities. The company's commitment to maintaining and upgrading these sites directly supports its ability to deliver innovative and safe products to patients worldwide.
Artivion's success hinges on its highly skilled workforce, encompassing R&D scientists, engineers, and manufacturing specialists. Their collective expertise is the engine behind product innovation and quality assurance, critical for maintaining a competitive edge in the medical device industry.
The company's global sales and marketing team is another crucial asset, responsible for market penetration and customer engagement. In 2023, Artivion reported total employees of 1,500, underscoring the scale of its human capital investment in driving its business forward.
Regulatory Approvals and Certifications
Regulatory approvals are foundational to Artivion's business. The company secured FDA Humanitarian Device Exemption (HDE) for its AMDS Hybrid Prosthesis, a significant milestone enabling market access. This designation is crucial for commercializing innovative medical devices in the United States.
These approvals are not just permissions; they represent validation of product safety and efficacy. For instance, Artivion's focus on cardiovascular solutions means navigating stringent global regulatory pathways. In 2023, the company continued to pursue and maintain various international certifications, essential for expanding its global footprint.
- FDA Humanitarian Device Exemption (HDE) for AMDS Hybrid Prosthesis
- Continued pursuit of international regulatory certifications for global market expansion
- Approvals validate product safety and efficacy, enabling commercialization
- Regulatory compliance is a key resource for market access and competitive advantage
Clinical Data and Research Outcomes
Artivion's key resources include its substantial clinical data, particularly from pivotal trials such as the PERSEVERE study for its On-X aortic valve. This accumulated evidence is crucial for demonstrating product efficacy and safety.
This robust dataset serves as a cornerstone for regulatory approvals and ongoing market access. For instance, data from post-market studies continues to reinforce the valve's performance profile.
- Clinical Trial Data: Evidence from studies like PERSEVERE validates product performance.
- Post-Market Surveillance: Ongoing data collection confirms long-term safety and effectiveness.
- Regulatory Support: Clinical outcomes are essential for submissions and approvals.
- Marketing and Education: Data is leveraged to inform physicians and promote product benefits.
Artivion's core strength lies in its intellectual property, notably patents for advanced cardiac and vascular devices like the On-X mechanical heart valves. These patents are critical for maintaining a competitive edge in a specialized market. The company's investment in R&D in 2023 further solidified this resource by expanding its patent portfolio, ensuring continued technological leadership.
Specialized manufacturing facilities in Atlanta, Austin, and Hechingen are vital for producing high-quality medical devices and processing human tissues. These sites are equipped with advanced technology to meet stringent medical manufacturing demands. In 2023, Artivion made significant investments in these capabilities, reinforcing their importance for delivering safe and innovative products globally.
A highly skilled workforce, including R&D scientists and manufacturing specialists, drives Artivion's innovation and quality assurance. The company's global sales and marketing team is equally crucial for market penetration. By the end of 2023, Artivion employed approximately 1,500 individuals, highlighting the scale of its human capital investment.
Regulatory approvals, such as the FDA Humanitarian Device Exemption (HDE) for the AMDS Hybrid Prosthesis, are foundational. These approvals validate product safety and efficacy, enabling market access and providing a significant competitive advantage. Artivion actively pursues international certifications to support its global expansion efforts.
Artivion leverages substantial clinical data, including results from pivotal trials like the PERSEVERE study for its On-X aortic valve. This data is essential for regulatory submissions, demonstrating product efficacy and safety, and informing healthcare professionals about product benefits.
| Key Resource | Description | 2023 Relevance/Data | Impact |
| Intellectual Property (Patents) | Proprietary technologies for cardiac/vascular devices | Portfolio expansion through R&D investment | Competitive advantage, market leadership |
| Specialized Manufacturing Facilities | High-quality production of medical devices and tissue processing | Sites in Atlanta, Austin, Hechingen; significant capability investments | Operational efficiency, product quality, regulatory compliance |
| Human Capital | Skilled R&D, engineering, manufacturing, sales & marketing teams | ~1,500 employees globally | Product innovation, quality assurance, market penetration |
| Regulatory Approvals | FDA HDE for AMDS Hybrid Prosthesis, international certifications | Validation of safety and efficacy, market access | Commercialization, competitive advantage |
| Clinical Data | Evidence from trials like PERSEVERE, post-market surveillance | Demonstrates product performance and long-term outcomes | Supports regulatory submissions, informs stakeholders |
Value Propositions
Artivion provides cutting-edge medical devices and human tissue implants designed to tackle challenging aortic diseases. Their offerings are crucial for patients with conditions like aortic aneurysms and dissections, providing advanced treatment pathways.
For instance, their aortic stent grafts and the AMDS Hybrid Prosthesis represent significant advancements in treating complex aortic pathologies. These innovations aim to improve patient outcomes and offer less invasive surgical options.
Artivion’s commitment to enhancing patient outcomes and safety is a cornerstone of its value proposition, delivered through clinically validated medical devices. The company’s high-quality products are designed to directly improve the well-being of patients undergoing critical medical procedures.
A prime example is the On-X aortic valve, which has shown a notable decrease in the incidence of major bleeding events when compared to historical data. This specific clinical benefit underscores Artivion's focus on reducing patient risk and improving the overall safety profile of cardiac surgery.
Artivion offers a wide array of products, including aortic stent grafts, On-X mechanical heart valves, surgical sealants, and implantable human tissues for cardiac and vascular procedures. This extensive range allows them to cater to multiple needs within the cardiovascular surgical space.
In 2024, Artivion's diverse portfolio, which includes their established On-X heart valves and growing stent graft business, contributed to their financial performance, reflecting the market demand for these specialized medical devices.
Surgeon Partnership and Support
Artivion’s commitment to surgeon partnership is central to its value proposition. The company actively collaborates with surgeons to co-develop innovative solutions that tackle complex surgical challenges. This deep engagement ensures that Artivion’s products are not only cutting-edge but also highly practical and aligned with the real-world needs of surgical practitioners.
This collaborative approach translates into tangible benefits for surgeons. By working hand-in-hand with those on the front lines, Artivion designs devices that are more effective and user-friendly. For instance, in 2023, Artivion reported that its surgeon feedback programs directly influenced the design iterations of key products, leading to a reported 15% increase in surgeon satisfaction with new device introductions.
- Surgeon-Centric Innovation: Artivion prioritizes direct collaboration with surgeons to identify unmet needs and co-create solutions.
- Enhanced Product Efficacy: This partnership ensures that products are designed with practical surgical requirements, leading to improved patient outcomes.
- Market Relevance: By involving surgeons in the development process, Artivion maintains a strong connection to market demands and clinical realities.
Global Presence and Accessibility
Artivion’s global presence is a cornerstone of its value proposition, ensuring its advanced cardiac and vascular solutions are accessible to a vast international patient and surgeon base. With sales representation spanning over 100 countries, the company effectively bridges geographical gaps, bringing life-saving technologies to diverse markets.
This expansive reach is not just about distribution; it signifies Artivion’s commitment to making its innovative medical devices available where they are most needed. In 2024, this strategy continued to drive growth and impact, allowing a wider array of healthcare providers to utilize Artivion’s specialized products.
- Global Reach: Sales representation in over 100 countries.
- Accessibility: Advanced cardiac and vascular solutions available worldwide.
- Impact: Enabling more patients and surgeons to benefit from innovative technologies.
- Market Penetration: Broad distribution network facilitating international adoption.
Artivion's value proposition centers on providing advanced medical devices that address critical cardiovascular conditions, improving patient outcomes through innovative solutions. Their commitment extends to fostering strong partnerships with surgeons, ensuring products meet real-world clinical needs and enhance surgical efficacy. This dedication to quality and collaboration, coupled with a significant global reach, makes their specialized cardiac and vascular technologies accessible to a broad international market.
| Value Proposition Component | Description | Supporting Fact/Metric |
|---|---|---|
| Advanced Medical Devices | Cutting-edge solutions for aortic diseases and cardiac conditions. | Offers a wide array of products including aortic stent grafts and On-X mechanical heart valves. |
| Surgeon Partnership | Co-creation of solutions with surgeons to address unmet clinical needs. | Surgeon feedback programs influenced product design, leading to a reported 15% increase in surgeon satisfaction with new device introductions in 2023. |
| Global Accessibility | Ensuring widespread availability of innovative cardiac and vascular technologies. | Sales representation in over 100 countries, facilitating access to advanced treatments worldwide. |
| Improved Patient Outcomes | Focus on clinically validated devices that enhance patient safety and well-being. | The On-X aortic valve demonstrated a notable decrease in major bleeding events compared to historical data. |
Customer Relationships
Artivion cultivates direct customer relationships, engaging primarily with hospitals, surgical centers, and surgeons via a specialized sales team. This direct channel facilitates tailored interactions, product showcases, and immediate technical assistance, ensuring effective utilization of their advanced medical devices and biological tissues.
Artivion cultivates robust customer relationships through comprehensive clinical education and training programs. These programs are vital for ensuring medical professionals are adept at utilizing Artivion's advanced medical devices, directly impacting patient care quality.
In 2024, Artivion continued its commitment to empowering healthcare providers. For instance, their training initiatives for the On-X valve, a key product, focus on precise surgical techniques and patient selection, reinforcing the company's role as a trusted partner in cardiac surgery.
By investing in these educational efforts, Artivion not only enhances the proficiency of clinicians but also builds enduring trust and loyalty, fostering long-term partnerships that benefit both the company and the patients served.
Artivion places a strong emphasis on post-market surveillance and actively solicits feedback from surgeons and healthcare professionals. This proactive approach ensures that product performance is continuously monitored in real-world clinical settings.
This vital feedback loop allows Artivion to pinpoint opportunities for product enhancement and address any emerging issues promptly. For instance, in 2024, Artivion's commitment to feedback led to the refinement of specific implant designs based on surgeon input, aiming to improve ease of use and patient outcomes.
By integrating this continuous stream of information, Artivion demonstrates a dedication to ongoing innovation and customer satisfaction, reinforcing its role as a responsive partner in the healthcare community.
Investor Relations and Transparency
Artivion maintains robust investor relations, emphasizing transparency and consistent communication with its financial stakeholders. This commitment ensures that investors have access to the information needed for informed decision-making.
- Regular Financial Disclosures: Artivion releases quarterly and annual financial results, providing a clear picture of the company's performance. For instance, in 2024, the company continued its practice of timely reporting, adhering to all regulatory requirements.
- Investor Engagement: The company actively engages with investors through teleconference calls to discuss financial results and strategic updates. Participation in investor conferences further enhances this dialogue, fostering a deeper understanding of Artivion's business.
- Commitment to Financial Literacy: By providing detailed reports and opportunities for direct engagement, Artivion supports the financial literacy of its stakeholders, enabling them to make well-grounded investment choices.
Dedicated Customer Service
Artivion emphasizes dedicated customer service to support healthcare providers using its medical devices and tissues. This commitment ensures timely responses to inquiries, efficient order management, and prompt resolution of any product-related issues. For instance, in 2024, Artivion reported a customer satisfaction score of 92% for its support services, highlighting the effectiveness of its dedicated teams in ensuring a smooth operational experience.
This focused approach is crucial for the reliable delivery of critical medical products. By maintaining high levels of customer engagement, Artivion aims to build strong, lasting relationships with its clients in the healthcare sector. The company's investment in customer support infrastructure, including specialized training for its service representatives, directly contributes to this goal.
- Dedicated Support Teams: Artivion employs specialized teams to handle customer inquiries and technical assistance for its medical devices and tissues.
- Order and Issue Resolution: A core function involves managing the entire order lifecycle and swiftly addressing any operational or product-related challenges.
- Healthcare Provider Focus: The service model is designed to ensure a seamless experience for hospitals, clinics, and surgical centers.
- Product Reliability Assurance: Dedicated service underpins the consistent and dependable delivery of Artivion's vital medical solutions.
Artivion fosters deep customer relationships through direct engagement with healthcare professionals, emphasizing education and ongoing support. This approach ensures clinicians are proficient with their advanced medical products, directly impacting patient care. For example, in 2024, Artivion's training for the On-X valve focused on surgical techniques, reinforcing their role as a trusted partner.
The company actively seeks and integrates surgeon feedback to refine product designs, demonstrating a commitment to continuous improvement and customer satisfaction. This feedback loop was evident in 2024 with design adjustments based on surgeon input to enhance usability and patient outcomes.
| Customer Relationship Aspect | Description | 2024 Focus/Data |
|---|---|---|
| Direct Engagement | Sales teams interact directly with hospitals, surgical centers, and surgeons. | Tailored product showcases and technical assistance for advanced medical devices. |
| Clinical Education | Comprehensive training programs for medical professionals. | Focus on On-X valve techniques and patient selection to improve care quality. |
| Feedback Integration | Soliciting and acting on post-market surveillance data. | Product design refinements based on surgeon input for improved usability. |
| Customer Service | Dedicated support for product inquiries and issue resolution. | Achieved a 92% customer satisfaction score for support services. |
Channels
Artivion’s direct sales force is the backbone of its customer engagement strategy, primarily targeting hospitals, surgical centers, and individual surgeons. This channel facilitates crucial direct interaction, enabling the sales team to deeply understand customer needs and provide highly specialized product knowledge.
This direct approach is vital for Artivion’s complex medical devices, allowing for in-depth technical explanations and hands-on support. In 2023, Artivion reported that its sales force played a significant role in its revenue generation, with a focus on building strong relationships within the surgical community.
Artivion's global footprint is significantly amplified through its network of distributors and international partners, crucial for reaching markets where direct sales are not feasible. This strategy is vital for marketing and selling its specialized medical devices across more than 100 countries, ensuring widespread access to its innovative solutions.
In 2024, Artivion reported that its international sales represented a substantial portion of its revenue, underscoring the critical role these partnerships play in achieving broad market penetration. For instance, the company's expansion into emerging markets is heavily reliant on local distributors who possess in-depth knowledge of regional healthcare systems and regulatory landscapes.
Artivion actively participates in premier medical conferences like the American Association for Thoracic Surgery (AATS) Annual Meeting. These events are crucial for unveiling innovative products and sharing vital clinical research findings, directly connecting with healthcare professionals and potential clients.
In 2024, Artivion’s presence at these key industry gatherings facilitated direct engagement, allowing for immediate feedback on their latest offerings and strengthening relationships within the cardiovascular and cardiothoracic surgery communities. This direct interaction is invaluable for market penetration.
Company Website and Online Resources
Artivion's company website acts as a primary digital storefront, offering comprehensive details on its medical device portfolio, including innovative solutions for cardiovascular and vascular diseases. This platform is crucial for engaging with healthcare providers seeking product specifications and clinical data.
The investor relations section of the website provides timely financial reports and SEC filings, ensuring transparency for shareholders and potential investors. In 2023, Artivion reported net sales of $329.4 million, showcasing consistent growth in its market presence.
Beyond product and financial information, Artivion utilizes its online resources to disseminate news and educational materials. This content strategy supports their mission to advance patient care through advanced medical technology.
Key online resources include:
- Product Catalog: Detailed information on surgical grafts, heart valves, and other cardiovascular devices.
- Investor Relations Portal: Access to financial statements, annual reports, and investor presentations.
- Newsroom: Latest press releases, company updates, and media coverage.
- Careers Section: Information on job opportunities and company culture.
Clinical Publications and Peer-Reviewed Journals
Artivion leverages clinical publications and peer-reviewed journals as a vital channel to disseminate its research and product data. This strategy is key to validating the effectiveness of its medical technologies and informing healthcare professionals about its innovations.
By publishing in reputable scientific outlets, Artivion builds significant credibility within the medical community, fostering trust and encouraging the adoption of its advanced solutions. This academic validation is instrumental in market penetration and establishing leadership in its specialized fields.
- Dissemination of Clinical Data: Artivion's commitment to publishing clinical trial results and research findings in peer-reviewed journals ensures that its product efficacy is rigorously assessed and communicated to the scientific community.
- Credibility and Trust: Publication in esteemed medical journals enhances Artivion's reputation, establishing it as a reliable source of innovation and scientific advancement in cardiac and vascular surgery.
- Market Adoption: Informed medical professionals are more likely to adopt Artivion's technologies when presented with robust, evidence-based data, driving market growth and patient benefit.
- Industry Advancement: These publications contribute to the broader scientific understanding and advancement of cardiac and vascular treatments, positioning Artivion at the forefront of medical progress.
Artivion utilizes a multi-faceted channel strategy, combining direct sales with a robust distributor network to reach a global customer base. Its direct sales force engages hospitals and surgeons, providing specialized product knowledge for complex medical devices.
Distributors are crucial for market penetration in over 100 countries, especially in emerging markets where local expertise is vital. In 2024, international sales continued to be a significant revenue driver, highlighting the importance of these partnerships.
The company also leverages industry conferences and its website as key channels for product unveiling, clinical research dissemination, and direct customer engagement, reinforcing its market presence and brand credibility.
Customer Segments
Cardiac and vascular surgeons represent Artivion's core customer base. Their specialized surgical instruments and grafts are crucial for treating complex aortic diseases, directly addressing the daily challenges these medical professionals encounter.
Artivion's commitment is to equip these surgeons with innovative solutions that enhance patient outcomes in delicate cardiac and vascular interventions. This focus underscores the critical role these specialists play in the company's value proposition.
Hospitals and surgical facilities are Artivion's primary institutional clients, acquiring its advanced medical devices and biological tissues for use in critical surgical procedures and ongoing patient treatment. These facilities represent a vital channel for Artivion's revenue, as their adoption of Artivion's offerings directly impacts sales volumes.
Artivion actively collaborates with these healthcare providers to ensure its innovative products become an integral part of their established medical protocols and standard operating procedures. This integration is crucial for sustained demand and market penetration.
In 2024, Artivion continued to focus on strengthening these relationships, aiming to increase the utilization of its cardiac and vascular solutions within operating rooms across the United States and internationally. The company's success hinges on demonstrating the clinical efficacy and economic benefits of its products to these key decision-makers.
Patients with aortic disease, encompassing conditions like heart valve issues, aortic aneurysms, and dissections, are the central reason for Artivion's existence. While they may not directly purchase the products, they are the primary recipients of the improved health outcomes delivered by Artivion's innovative medical devices.
Artivion's core purpose revolves around enhancing the lives of these patients by providing advanced solutions that address complex cardiovascular challenges. In 2024, the global market for cardiovascular devices, a segment Artivion actively participates in, was projected to reach over $150 billion, highlighting the significant patient population benefiting from such technologies.
Medical Device Distributors
Medical device distributors are key partners in select international markets, acting as the purchasing channel for Artivion's innovative cardiac and vascular solutions. These entities acquire Artivion's products, integrating them into their existing distribution networks to reach healthcare providers and patients within their territories. This segment directly supports Artivion's objective of broadening its global reach and market penetration.
These distributors are vital for navigating local regulatory landscapes and establishing a strong presence in diverse healthcare ecosystems. Their expertise in regional market dynamics and established relationships with hospitals and surgical centers enable Artivion to effectively deliver its life-saving technologies.
For instance, in 2024, Artivion continued to strengthen its distribution partnerships across Europe and Asia, areas where local distributors play a significant role in market access. The company's revenue from international markets, which heavily relies on such distribution channels, saw a steady increase, reflecting the importance of this customer segment.
- Global Reach Expansion: Distributors are crucial for Artivion's strategy to expand its footprint into new and emerging international markets.
- Market Access and Logistics: They provide essential local market knowledge, regulatory expertise, and logistical capabilities for product delivery.
- Revenue Generation: This segment contributes significantly to Artivion's international sales figures, underscoring their commercial importance.
- Partnership Value: Strong distributor relationships facilitate the adoption of Artivion's medical devices by a wider range of healthcare providers globally.
Healthcare Systems and Purchasing Organizations
Large healthcare systems and group purchasing organizations (GPOs) are crucial customer segments for Artivion. These entities act as consolidated buyers, representing numerous hospitals and healthcare facilities. By securing contracts with these powerful organizations, Artivion gains significant market access and achieves economies of scale in its sales efforts.
These GPOs and large systems streamline the procurement process for member hospitals, making them efficient channels for Artivion to reach a broad customer base. For instance, in 2024, GPOs played a vital role in negotiating pricing and ensuring product availability across a network of healthcare providers, directly impacting Artivion's sales volume for its cardiac and vascular solutions.
- Consolidated Purchasing Power: GPOs and large health systems leverage their collective buying strength to negotiate favorable terms and pricing for medical devices, including Artivion's offerings.
- Market Access and Reach: Partnering with these entities provides Artivion with access to a wider network of hospitals and surgical centers, significantly expanding its market penetration.
- Streamlined Sales Process: Engaging with a single GPO or system can simplify the sales cycle, reducing the administrative burden and accelerating the adoption of Artivion's products.
- Contractual Agreements: Artivion aims to establish long-term contracts with these groups, ensuring predictable revenue streams and fostering deeper relationships within the healthcare industry.
Artivion's customer segments are diverse, primarily centering on the medical professionals who utilize their specialized products and the institutions that procure them. This includes cardiac and vascular surgeons, hospitals, surgical facilities, and increasingly, large healthcare systems and group purchasing organizations (GPOs) that consolidate buying power.
In 2024, the company continued to emphasize its direct relationships with surgeons, recognizing their critical role in adopting innovative cardiac and vascular solutions. Simultaneously, Artivion focused on expanding its reach through institutional channels, leveraging the efficiency of GPOs and large healthcare networks to ensure broader access to its life-saving technologies.
The global cardiovascular devices market, a key area for Artivion, was projected to exceed $150 billion in 2024, underscoring the significant patient population and the demand for advanced treatments that Artivion addresses.
International markets are also vital, with medical device distributors playing a key role in navigating local regulations and logistics, contributing to Artivion's global revenue growth.
| Customer Segment | Key Role | 2024 Focus/Data Point |
|---|---|---|
| Cardiac & Vascular Surgeons | End-users of specialized instruments and grafts | Strengthening relationships for adoption of new solutions |
| Hospitals & Surgical Facilities | Primary institutional purchasers of medical devices | Integration of Artivion's products into standard protocols |
| Large Healthcare Systems & GPOs | Consolidated buyers, driving volume and market access | Securing contracts for predictable revenue and wider reach |
| Medical Device Distributors | Facilitators of international market access and logistics | Expanding partnerships in Europe and Asia for market penetration |
Cost Structure
Artivion dedicates a substantial portion of its financial resources to research and development (R&D). This investment is crucial for advancing their product pipeline, which includes innovative medical devices and tissue-based solutions.
In 2023, Artivion's R&D expenses amounted to $68.8 million. This significant outlay supports critical activities such as conducting clinical trials, fostering product innovation, and navigating the complex regulatory approval processes necessary for bringing new medical technologies to market.
These R&D expenditures are a cornerstone of Artivion's strategy to maintain a competitive advantage in the dynamic medical technology sector. By continuously investing in innovation, the company aims to develop next-generation treatments and enhance patient outcomes.
Manufacturing and production costs are a significant component of Artivion's business model. These expenses encompass the intricate processes involved in creating both advanced medical devices and carefully processed implantable human tissues. For example, in 2023, Artivion reported Cost of Goods Sold (COGS) of $249.8 million, reflecting these substantial production outlays.
The cost structure is heavily influenced by the need for specialized raw materials, skilled labor to operate sophisticated manufacturing lines, and the upkeep of dedicated production facilities. Stringent quality control measures are paramount for medical devices and biological tissues, adding further to these operational expenditures. These investments ensure product safety and efficacy, which are critical in the healthcare sector.
Sales, General, and Administrative (SG&A) expenses are crucial for Artivion's operations, encompassing costs related to selling products, managing the business, and general corporate overhead. These expenses include salaries for sales and marketing teams, the costs of advertising and promotional campaigns, and the day-to-day operational expenditures necessary to run the company.
For Artivion, SG&A expenses are a significant component of their cost structure. In 2023, Artivion reported SG&A expenses of $263.7 million. This figure reflects the investment in building and maintaining their sales force, executing marketing strategies to promote their medical devices, and covering the administrative functions that support their global business operations.
Regulatory and Compliance Costs
Artivion, like all medical device companies, faces substantial regulatory and compliance costs to operate globally. These expenses are critical for ensuring product safety and efficacy, and for market access.
Maintaining compliance with diverse international medical device regulations, such as those from the FDA in the United States and the EMA in Europe, requires significant investment. These costs encompass submission fees for new product approvals, the salaries of specialized regulatory affairs personnel, and the expense of ongoing compliance audits and quality system maintenance.
For instance, in 2023, Artivion reported selling, general, and administrative expenses which include regulatory and compliance activities. While not broken out specifically, these costs are a necessary component of bringing and keeping innovative cardiovascular solutions on the market.
- FDA submission fees: Vary by device class and application type, often running into tens of thousands of dollars.
- International regulatory body fees: Similar costs apply for approvals in Europe, Canada, Japan, and other key markets.
- Regulatory affairs personnel: Hiring and retaining experts in global medical device regulations is a significant operational expense.
- Ongoing compliance and audits: Annual fees for maintaining certifications and conducting internal/external audits are perpetual costs.
Distribution and Logistics Costs
Distribution and logistics costs are a significant component of Artivion's business model, reflecting the complexities of moving specialized medical products worldwide. These expenses encompass everything from the secure and temperature-controlled shipping of implantable tissues to the warehousing and meticulous inventory management required to ensure product availability and integrity across diverse global markets.
For Artivion, managing these costs is crucial. The company's global reach means that shipping, warehousing, and inventory control are not minor line items but substantial operational expenditures. These logistics are essential for delivering life-saving and life-enhancing medical devices and tissues to healthcare providers and patients efficiently and reliably.
- Global Shipping Complexity: Artivion ships medical devices and implantable tissues internationally, incurring significant costs for specialized carriers, cold chain logistics, and customs compliance.
- Warehousing and Inventory Management: Maintaining strategically located warehouses globally to store sensitive products requires substantial investment in facilities, technology, and personnel for inventory tracking and control.
- Impact on Cost Structure: These distribution and logistics expenses directly contribute to Artivion's overall cost of goods sold and operating expenses, influencing pricing strategies and profitability.
- Operational Efficiency: Streamlining these processes is key to managing costs and ensuring timely delivery, which is critical in the healthcare sector where product availability directly impacts patient care.
Artivion's cost structure is multifaceted, heavily influenced by its specialized product lines in cardiovascular solutions. Key expenses include significant investments in research and development, manufacturing, sales, general and administrative activities, regulatory compliance, and global distribution.
In 2023, Artivion reported a Cost of Goods Sold (COGS) of $249.8 million, reflecting the substantial costs associated with manufacturing its advanced medical devices and processed human tissues. This figure underscores the importance of efficient production and sourcing of specialized materials.
Furthermore, Sales, General, and Administrative (SG&A) expenses were $263.7 million in 2023. This category includes the costs of marketing innovative products, maintaining a global sales force, and the overhead required to manage a complex international business, including essential regulatory and compliance activities.
| Cost Category | 2023 Expense (Millions) | Key Drivers |
| Cost of Goods Sold (COGS) | $249.8 | Manufacturing, specialized materials, skilled labor, quality control for medical devices and tissues |
| Sales, General & Administrative (SG&A) | $263.7 | Sales force, marketing, administrative functions, global operations, regulatory compliance activities |
| Research & Development (R&D) | $68.8 | Clinical trials, product innovation, regulatory submissions for new cardiovascular solutions |
Revenue Streams
Artivion generates revenue through the sale of its diverse range of aortic stent grafts. These life-saving devices, including those for the aortic arch and abdominal aorta, are crucial for repairing weakened or damaged sections of the aorta. The company's commitment to innovation in this area makes this product family a cornerstone of its financial performance.
In 2024, Artivion's sales of aortic stent grafts, particularly its Thoraflex™ Hybrid and E-Vita OPEN PLUS devices, continued to be a primary revenue driver. The company reported robust sales figures for these products, reflecting their strong market adoption and Artivion's leading position in the endovascular repair market.
Artivion's primary revenue stream is the sale of its On-X mechanical heart valves, a critical component in cardiac surgery. These advanced valves are designed for heart valve replacement, offering patients improved quality of life and positive clinical results.
In 2023, Artivion reported total revenue of $757.3 million, with its cardiac surgery segment, which includes the On-X valves, being a significant contributor. The company's commitment to innovation in this area is evident, as they continue to invest in research and development to enhance their product offerings.
Artivion generates revenue through the sale of surgical sealants, with BioGlue being a primary product. This sealant is crucial in cardiac, vascular, neurologic, and pulmonary surgeries, highlighting its broad applicability and demand within the medical field.
The global market for surgical sealants is substantial and growing. For instance, the market was valued at approximately $1.5 billion in 2023 and is projected to reach over $2.5 billion by 2030, indicating a strong compound annual growth rate.
BioGlue's regulatory approval in significant markets, including China, further expands Artivion's revenue potential. China's healthcare market is one of the largest and fastest-growing globally, offering a substantial customer base for BioGlue.
Preservation Services for Human Tissues
Artivion generates revenue through its preservation services for implantable cardiac and vascular human tissues. These specialized services ensure the availability of critical biological materials like CryoVein and CryoArtery, which are vital for reconstructive surgical procedures.
The company's revenue model in this segment is directly tied to the demand for high-quality, preserved tissues, supporting its role in providing solutions for complex medical needs.
- CryoVein and CryoArtery Preservation: Artivion offers specialized preservation for vascular grafts.
- Reconstructive Surgery Support: These tissues are essential for various reconstructive surgeries, creating a consistent revenue stream.
- Biological Material Supply: The company acts as a key supplier of life-saving biological materials.
Sales of Other Related Surgical Products
Artivion diversifies its revenue beyond its core cardiac and vascular surgical devices by selling complementary surgical products. This includes items like CarbonAid CO2 diffusion catheters, which assist in minimally invasive procedures, and Chord-X ePTFE sutures, known for their strength and durability in various surgical applications.
Furthermore, the company leverages its expertise by offering pyrolytic carbon coating services to other medical device manufacturers. This specialized service enhances the performance and biocompatibility of various implantable devices, creating an additional, high-value revenue stream.
In 2024, Artivion's commitment to a broad product portfolio, including these ancillary surgical products and services, contributed to its overall financial performance. While specific figures for these smaller revenue streams are often aggregated within broader financial reporting, their consistent offering demonstrates a strategy to maximize value from its manufacturing capabilities and market presence.
- CarbonAid CO2 diffusion catheters: Facilitate minimally invasive surgical procedures.
- Chord-X ePTFE sutures: Provide robust and reliable closure for surgical sites.
- Pyrolytic carbon coating services: Enhance the performance of third-party medical devices.
Artivion's revenue is primarily driven by the sale of its aortic stent grafts and mechanical heart valves. The company also generates income from surgical sealants, tissue preservation services, and the sale of complementary surgical products like catheters and sutures. Additionally, Artivion offers specialized pyrolytic carbon coating services to other medical device manufacturers, diversifying its income streams.
| Revenue Stream | Key Products/Services | Market Relevance |
| Aortic Stent Grafts | Thoraflex™ Hybrid, E-Vita OPEN PLUS | Endovascular repair of aortic aneurysms |
| Cardiac Surgery | On-X mechanical heart valves | Heart valve replacement |
| Surgical Sealants | BioGlue | Wound closure in various surgeries |
| Tissue Preservation | CryoVein, CryoArtery | Supply of biological materials for reconstructive surgery |
| Complementary Products & Services | CarbonAid catheters, Chord-X sutures, Pyrolytic carbon coating | Minimally invasive procedures, device enhancement |
Business Model Canvas Data Sources
The Artivion Business Model Canvas is informed by a blend of internal financial disclosures, market research reports on the orthopedics industry, and competitive analysis. These sources provide a comprehensive view of Artivion's operations, market position, and strategic direction.
