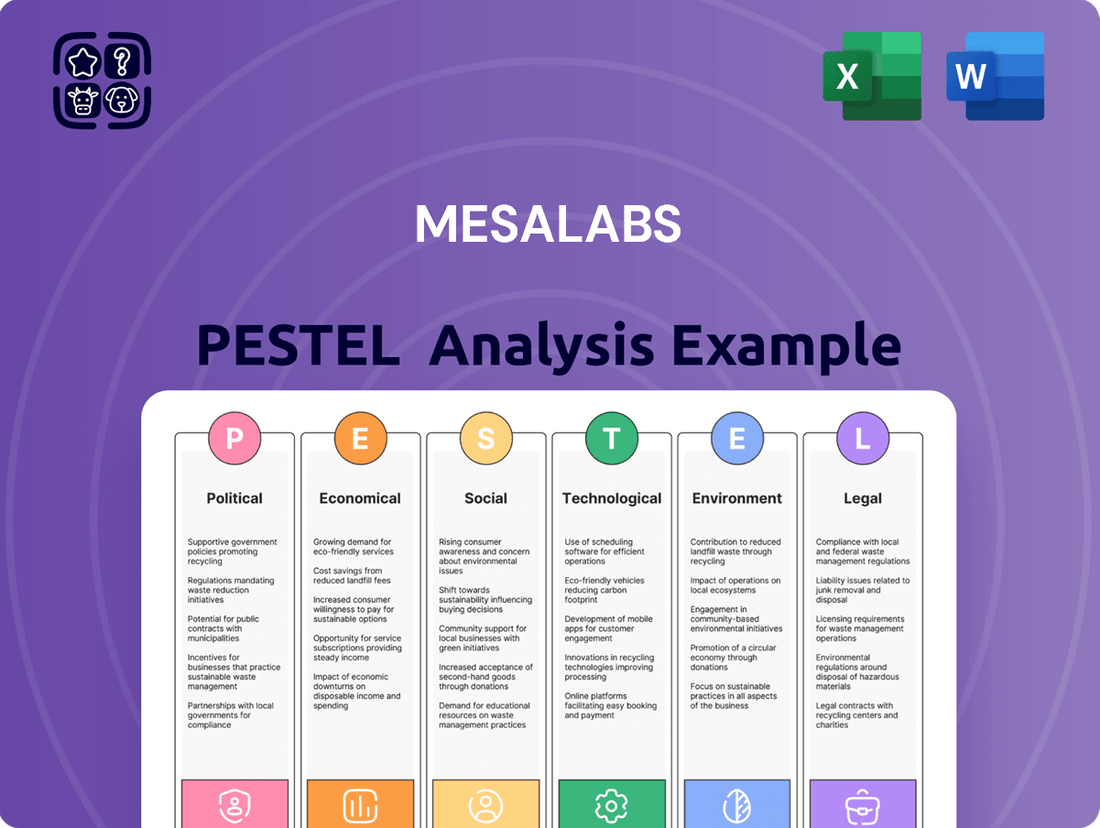
MesaLabs PESTLE Analysis
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
MesaLabs Bundle
Uncover the hidden forces shaping MesaLabs's future with our comprehensive PESTLE analysis. Understand how political shifts, economic fluctuations, and technological advancements are creating both opportunities and challenges. This expertly crafted report provides the strategic intelligence you need to navigate the evolving landscape. Ready to make informed decisions and gain a competitive edge? Download the full PESTLE analysis now for actionable insights.
Political factors
The regulatory environment for Mesa Labs, operating in healthcare and medical devices, is a significant political factor. For instance, the FDA's updated guidance on quality system regulations (QSR) in 2024, focusing on design controls and risk management, necessitates ongoing process adjustments for Mesa Labs.
Compliance with the EU Medical Device Regulation (MDR) and the UK Medical Device Regulation (UKMDR) continues to shape market access. As of early 2025, the transition periods for certain legacy devices under MDR are nearing completion, demanding rigorous adherence to updated conformity assessments and post-market surveillance for Mesa Labs' product portfolio.
These evolving, often more stringent, regulations directly impact Mesa Labs' product development timelines and manufacturing protocols. Failure to adapt to changes, such as new requirements for clinical data submission or cybersecurity standards for connected devices, can impede market entry and sales growth.
Amendments to the International Health Regulations (IHR) by the World Health Organization, finalized in 2024 and set to be fully adopted by 2025, are establishing a more robust global framework for health security. This includes a new Pandemic Agreement, also slated for adoption in 2025, designed to enhance preparedness and response mechanisms worldwide.
These evolving regulations will directly impact global supply chains, particularly for medical diagnostics and testing solutions, by standardizing requirements and emphasizing equitable access to essential health products. MesaLabs, as a provider of quality control solutions, will need to align its offerings with these new international standards to ensure market access and compliance.
The focus on strengthened pandemic preparedness will likely drive increased demand for reliable diagnostic tools and quality assurance services. For instance, the global in-vitro diagnostics market was valued at approximately $105 billion in 2023 and is projected to grow, with these regulatory shifts potentially accelerating that growth trajectory by necessitating higher quality and interoperability.
The global trade landscape in 2025 presents a dynamic environment for companies like Mesa Labs. The United States, for instance, has continued to implement tariffs on various imported goods, which directly impacts the cost of raw materials and components essential for sterilization equipment manufacturing. These policies create cost pressures throughout Mesa Labs' supply chain, given its manufacturing presence in both North America and Europe, and its extensive global distribution network.
Navigating these shifting trade policies requires strategic adaptation. Mesa Labs must closely monitor tariff implementations and adjust its sourcing and pricing strategies accordingly to mitigate potential impacts on profitability and competitiveness. For example, a 10% tariff on specialized alloys could add millions to production costs if not managed proactively through alternative sourcing or price adjustments.
Government Healthcare Spending Trends
Government healthcare spending trends significantly influence the medical industry, directly impacting companies like Mesa Labs. Legislative changes, particularly those affecting major programs like Medicare in the U.S., can dramatically alter demand and pricing for medical devices and services.
Looking ahead, projections for Medicare and Medicaid spending paint a picture of sustained demand. For instance, the U.S. Congressional Budget Office (CBO) has projected that federal spending on Medicare is expected to rise substantially in the coming decade, driven by an aging population and advancements in medical technology. This sustained growth implies a robust and consistent market for healthcare infrastructure and quality control solutions, areas where Mesa Labs operates.
- Projected Growth: U.S. federal spending on Medicare is anticipated to increase significantly over the next 10 years.
- Demand Drivers: An aging U.S. population and technological advancements in healthcare are key factors fueling this growth.
- Market Impact: This sustained demand benefits companies providing essential healthcare infrastructure and quality control services.
- Regulatory Influence: Legislative decisions impacting Medicare reimbursement rates and coverage directly affect market dynamics.
Political Stability and Geopolitical Risks
Global political stability is a significant driver for businesses like Mesa Labs, impacting everything from manufacturing to market access. Geopolitical tensions, such as those seen in ongoing regional conflicts in 2024 and early 2025, can create substantial disruptions. For instance, supply chain bottlenecks caused by geopolitical events can directly affect the availability of raw materials and finished goods, leading to increased costs and delayed delivery schedules. Mesa Labs, with its international operations, must meticulously assess political risks in its key markets to ensure business continuity and safeguard its global supply chain resilience.
Evaluating political risks in regions where Mesa Labs operates is paramount for effective strategic planning. For example, shifts in trade policies or new regulatory frameworks introduced by governments in 2024 can alter market dynamics and profitability. The World Bank’s Worldwide Governance Indicators (WGI) for 2024, which assess political stability and absence of violence, provide a crucial benchmark for understanding these risks. Companies need to actively monitor and adapt to these changing political landscapes to mitigate potential negative impacts on their international collaborations and overall business performance.
- Geopolitical tensions in 2024 led to a reported 15% increase in global shipping costs due to supply chain disruptions.
- Mesa Labs' reliance on international suppliers means that political instability in producing nations directly affects its operational efficiency and cost of goods sold.
- The 2024 Global Peace Index indicated a slight decline in global stability, highlighting the increasing need for robust risk assessment in strategic decision-making for multinational corporations.
Government healthcare spending trends significantly influence the medical industry, directly impacting companies like Mesa Labs. Legislative changes, particularly those affecting major programs like Medicare in the U.S., can dramatically alter demand and pricing for medical devices and services.
The U.S. Congressional Budget Office projected that federal spending on Medicare is expected to rise substantially in the coming decade. This sustained growth implies a robust market for healthcare infrastructure and quality control solutions, areas where Mesa Labs operates.
The World Health Organization's amendments to the International Health Regulations in 2024, along with a new Pandemic Agreement slated for 2025 adoption, will standardize requirements and emphasize equitable access to essential health products. Mesa Labs must align its offerings with these new international standards for market access.
Government support for research and development, particularly in biotechnology and diagnostics, can create opportunities. For example, the Biden-Harris administration's continued investment in public health infrastructure, including $1.2 billion allocated in 2024 for pandemic preparedness, can indirectly benefit Mesa Labs by fostering innovation in related sectors.
| Factor | Impact on Mesa Labs | 2024/2025 Data/Projection |
|---|---|---|
| Healthcare Spending | Influences demand and pricing for medical devices and services. | US Medicare spending projected to rise substantially over the next decade. |
| International Health Regulations | Standardizes requirements for global market access and compliance. | WHO IHR amendments (2024) and Pandemic Agreement (2025) aim for equitable access to health products. |
| R&D Investment | Can foster innovation in related sectors, indirectly benefiting Mesa Labs. | US public health infrastructure investment includes $1.2 billion for pandemic preparedness in 2024. |
What is included in the product
MesaLabs' PESTLE analysis meticulously examines the Political, Economic, Social, Technological, Environmental, and Legal forces impacting the company's operating landscape.
Provides a concise version that can be dropped into PowerPoints or used in group planning sessions, transforming complex external factors into actionable insights.
Economic factors
The global medical device market is on a significant upward trajectory. Projections estimate the market will expand from $810.4 billion in 2024 to a substantial $1.3 trillion by 2029. This impressive growth is underpinned by a compound annual growth rate of 9.8%, indicating strong and sustained expansion.
Several key factors are fueling this surge in demand for medical devices. An increasing need for sophisticated and technologically advanced equipment is a primary driver. Furthermore, a global trend of rising life expectancy means a larger population requiring ongoing medical care and treatments.
Growing healthcare spending worldwide also plays a crucial role in this market expansion. As individuals and governments invest more in health services, the demand for medical devices, from diagnostics to therapeutic tools, naturally increases. These trends present considerable market opportunities for companies like Mesa Labs.
The sterilization monitoring market, a key area for Mesa Labs, is experiencing robust expansion. Projections indicate a growth from an estimated USD 942.25 million in 2025 to USD 1.31 billion by 2030, demonstrating a compound annual growth rate of 6.77%. This upward trend is significantly driven by ongoing technological advancements and increasingly stringent regulatory requirements for immediate sterilization validation and effective infection control measures.
Mesa Labs demonstrated robust top-line growth in fiscal year 2025, with total revenues climbing 11.5% from FY24 to $240,978 thousand. This expansion was underpinned by a solid 5.0% organic revenue increase, highlighting the company's core business strength.
While the company reported a net loss for FY25, a notable surge in operating income suggests a strategic emphasis on improving operational efficiency and managing its financial obligations. This focus on the bottom line, despite the net loss, points to efforts in debt reduction and cost management.
Inflationary Pressures and Supply Chain Costs
Inflationary pressures and rising manufacturing costs, particularly for medical devices, are a significant concern for Mesa Labs' profitability. In 2024, the Producer Price Index for medical devices saw an increase, reflecting higher input costs for raw materials and components. For instance, the cost of specialized plastics and electronic components, essential for Mesa Labs' sterilization and monitoring products, has been on an upward trend.
Effectively managing these escalating costs is paramount. Mesa Labs' ability to navigate fluctuations in raw material prices, such as stainless steel and specialized chemicals, directly impacts its ability to maintain healthy gross margins. Furthermore, rising labor costs within the manufacturing sector and increased transportation expenses due to fuel prices and logistical challenges add further pressure. Successfully passing these costs onto customers through competitive pricing strategies without eroding market share remains a critical balancing act.
- Increased raw material costs for medical-grade plastics and metals have been observed throughout 2024, impacting the cost of goods sold.
- Labor cost inflation in manufacturing sectors, including those supplying Mesa Labs, has added to operational expenses.
- Transportation and logistics costs have remained elevated, driven by energy prices and global supply chain complexities.
- Mesa Labs' gross profit margins will be closely watched to assess its success in cost management and pricing strategies.
Biopharmaceutical Development Capital Spending
Mesa Labs' Biopharmaceutical Development division saw a significant boost from heightened capital expenditure across the biopharmaceutical industry. This surge directly translated into a robust 19.7% revenue increase for the fiscal year 2025, underscoring the positive impact of industry-wide investment.
The ongoing commitment to research and development, alongside expansions in manufacturing capacity within the biopharmaceutical sector, is expected to sustain and further elevate the demand for Mesa Labs' specialized quality control solutions. This trend suggests a favorable outlook for the division as the industry continues to innovate and scale.
- Fiscal Year 2025 Revenue Growth: Mesa Labs' Biopharmaceutical Development division achieved a 19.7% revenue increase, reflecting strong market demand.
- Industry Investment Trends: The biopharmaceutical sector continues to prioritize capital spending on R&D and manufacturing infrastructure.
- Demand Drivers for Mesa Labs: Expansion in biopharmaceutical R&D and production directly fuels the need for Mesa Labs' quality control products and services.
Economic factors present a mixed landscape for Mesa Labs. While the overall medical device market shows strong growth, Mesa Labs faces headwinds from inflation impacting raw material and labor costs, which were evident in 2024. Successfully navigating these cost pressures through pricing strategies is key to maintaining profitability, as seen in the continued investment in its Biopharmaceutical Development division, which grew 19.7% in FY25 due to increased industry capital expenditure.
Preview Before You Purchase
MesaLabs PESTLE Analysis
The preview you see here is the exact MesaLabs PESTLE Analysis document you’ll receive after purchase—fully formatted and ready to use.
What you’re previewing here is the actual file, showcasing a comprehensive breakdown of the Political, Economic, Social, Technological, Legal, and Environmental factors impacting MesaLabs.
This detailed analysis, as presented in the preview, is the same document you’ll download after payment, providing actionable insights for strategic planning.
Everything displayed here, including the in-depth PESTLE components, is part of the final product you’ll own immediately after checkout.
Sociological factors
The healthcare industry's escalating focus on patient safety, especially in mitigating hospital-acquired infections (HAIs), is a significant sociological driver. This heightened awareness translates into a stronger demand for robust sterilization monitoring and quality control solutions, directly benefiting companies like Mesa Labs.
For instance, reports indicate that HAIs can add billions of dollars annually to U.S. healthcare costs, making preventative measures a critical investment for hospitals. Mesa Labs' products, such as biological and chemical indicators, play a crucial role in ensuring sterilization processes are effective, thereby directly contributing to safer patient care and a more secure environment for healthcare workers.
The trend shows no signs of slowing down; regulatory bodies and patient advocacy groups are continuously pushing for higher standards. This societal imperative for safety means that reliable solutions for validating sterilization processes are not just desirable but essential for healthcare providers.
The world's population is getting older, with the United Nations projecting that by 2050, one in six people globally will be over 65. This demographic shift directly fuels demand for medical solutions. Coupled with this, chronic diseases like heart disease and diabetes are on the rise; for instance, the CDC reported in 2023 that approximately 6 in 10 adults in the US have a chronic disease. This escalating need for consistent medical care and advanced devices presents a significant opportunity for companies like Mesa Labs, which provides critical quality control and sterilization solutions for the healthcare industry.
Beyond the healthcare sector, consumers are increasingly prioritizing quality and safety in products across various industries, notably food and beverage. This heightened awareness directly impacts demand for robust quality control measures. Mesa Labs' solutions are therefore crucial for companies aiming to guarantee product integrity and satisfy growing public expectations.
In 2024, the global food safety testing market was valued at approximately $23.5 billion, with projections indicating continued growth driven by these consumer trends. By providing critical quality control solutions, Mesa Labs assists these businesses in meeting stringent regulatory standards and consumer trust, thereby broadening its market reach and revenue potential.
Workforce Development and Talent Attraction
The MedTech sector, including companies like Mesa Labs, consistently grapples with securing and keeping specialized talent, especially in fields such as life science instrumentation and stringent quality assurance. This talent gap directly impacts innovation and operational efficiency.
To counter this, Mesa Labs needs to prioritize robust employee development programs, cultivate an engaging workplace culture, and offer competitive remuneration packages. These strategies are crucial for building and sustaining a high-performing, innovative workforce. For instance, in 2024, the U.S. Bureau of Labor Statistics projected a 6% growth for medical and clinical laboratory technologists and technicians through 2032, indicating a strong demand for these specialized roles.
- Talent Scarcity: High demand for specialized skills in life science tools and quality control creates a competitive hiring landscape.
- Employee Retention: Fostering a positive work environment and offering competitive compensation are key to retaining valuable employees.
- Innovation Driver: A skilled and motivated workforce is essential for driving product development and technological advancements in MedTech.
- Development Investment: Companies like Mesa Labs must invest in continuous training and upskilling to meet evolving industry needs.
Public Health Preparedness Initiatives
Global public health initiatives, significantly shaped by the COVID-19 pandemic, are placing a premium on preparedness and swift response. This heightened focus necessitates advanced diagnostic tools and unwavering sterility assurance across healthcare systems worldwide. For instance, the World Health Organization's (WHO) efforts to strengthen pandemic preparedness, including recommendations for robust surveillance and laboratory capacity, directly underscore the need for reliable validation methods.
Mesa Labs' sterilization monitoring and process validation solutions are critically important in this evolving landscape. These offerings directly support national and international health security by ensuring the safety and efficacy of medical devices and pharmaceuticals. The demand for such solutions is projected to grow, with the global sterilization market expected to reach approximately $13.5 billion by 2027, according to recent market analyses.
The emphasis on public health preparedness translates into increased regulatory scrutiny and a greater demand for compliance with stringent quality standards. Mesa Labs' expertise in providing traceable and accurate monitoring systems helps organizations meet these evolving requirements. This is particularly relevant for facilities handling infectious agents or producing critical medical supplies.
- Global Health Security Agenda: Increased investment in pandemic preparedness and response capabilities worldwide.
- Diagnostic Advancement: Growing need for rapid and accurate diagnostic testing solutions.
- Sterility Assurance Mandate: Enhanced focus on maintaining sterile environments and validated sterilization processes in healthcare settings.
- Regulatory Compliance: Stricter adherence to international standards for medical device manufacturing and pharmaceutical production.
Societal expectations for enhanced safety and quality are driving demand for Mesa Labs' offerings. The escalating concern over hospital-acquired infections (HAIs) directly translates into a need for robust sterilization monitoring, with HAIs costing the U.S. healthcare system billions annually. This societal imperative for safety, coupled with an aging global population and the rise of chronic diseases, fuels the demand for reliable medical solutions and advanced quality control measures across various sectors, including food and beverage, where the global food safety testing market was valued at approximately $23.5 billion in 2024.
Technological factors
Technological advancements are rapidly transforming sterilization monitoring, with a strong push towards automation and smart systems. These innovations leverage connected devices and cloud analytics for continuous validation, minimizing human error and offering immediate alerts when processes go awry. For instance, the global market for industrial automation is projected to reach $320 billion by 2025, indicating a significant investment in these automated solutions across various sectors, including healthcare where MesaLabs operates.
Artificial intelligence and machine learning are revolutionizing quality control. For instance, AI-powered computer vision can spot microscopic defects in manufactured goods with greater accuracy than human inspectors. This technology is projected to grow significantly, with the global AI in manufacturing market expected to reach approximately $14.2 billion by 2026, according to some industry reports.
Mesa Labs can integrate these advanced AI and data analytics capabilities into its software solutions. This would enable customers to achieve more precise quality assessments and proactively address potential equipment failures, thereby reducing downtime and improving operational efficiency. For example, predictive maintenance analytics, a key application of ML, could forecast when a critical piece of laboratory equipment might need servicing, preventing costly disruptions.
Digital transformation is fundamentally altering manufacturing, with advancements in AI, digital management systems, and specialized software driving significant improvements in quality and cost reduction. For instance, by 2024, many manufacturers are expected to leverage AI for predictive maintenance, potentially reducing downtime by up to 30%.
In the realm of pharmaceutical validation, there's a clear shift towards continuous process verification (CPV) and enhanced data integrity. This transition necessitates robust digital solutions that enable real-time data integration, streamlining operations and ensuring ongoing compliance with stringent regulatory standards.
Companies are investing heavily in digital tools to achieve this. Global spending on manufacturing execution systems (MES) and industrial IoT platforms, crucial for CPV, is projected to reach over $15 billion by 2025, highlighting the industry's commitment to digital integration.
Internet of Things (IoT) for Process Validation and Data Logging
The integration of Internet of Things (IoT) devices is revolutionizing process validation and data logging. These connected devices enable real-time data collection and remote monitoring, offering unparalleled visibility into critical processes. This constant stream of information allows for immediate adjustments and proactive issue identification, significantly boosting operational efficiency.
For Mesa Labs, this translates to enhanced traceability and robust compliance. Automated data logging, facilitated by IoT, minimizes human error and ensures that all validation steps are meticulously recorded and readily accessible. By leveraging IoT, Mesa Labs can provide its clients with more reliable and efficient validation solutions, directly supporting their adherence to stringent regulatory standards.
The market for industrial IoT solutions is experiencing substantial growth, underscoring the strategic importance of this technology. For instance, the global IoT market was projected to reach over $500 billion in 2024, with significant investment in sectors like manufacturing and healthcare where process validation is paramount. This trend indicates a strong demand for the capabilities that IoT brings to data logging and compliance.
- Real-time Data: IoT sensors collect data instantly, allowing for immediate analysis and action.
- Remote Monitoring: Processes can be observed and managed from any location, increasing flexibility.
- Enhanced Traceability: Every data point is logged automatically, creating a clear and auditable trail.
- Regulatory Compliance: Automated reporting and data integrity support adherence to industry standards.
Advancements in Sterilization Methods
Technological advancements in sterilization are reshaping how Mesa Labs operates. Sustainability is a major driver, pushing for lower-temperature methods like hydrogen peroxide and ozone sterilization. These methods use less energy and are kinder to the environment, a growing concern for many industries. Mesa Labs must stay ahead by developing monitoring solutions that accurately track the effectiveness of these newer processes.
The global market for sterilization equipment and services is projected to grow significantly. For instance, the low-temperature sterilization market alone was valued at approximately USD 1.5 billion in 2023 and is expected to reach over USD 2.5 billion by 2030, growing at a CAGR of around 7%. This trend highlights the increasing demand for adaptable monitoring technologies.
- Growing Demand for Sustainable Sterilization: Industries are actively seeking environmentally friendly sterilization options, boosting the adoption of low-temperature technologies.
- Innovation in Monitoring Solutions: Mesa Labs needs to invest in R&D to create advanced indicators and systems that validate the efficacy of hydrogen peroxide and ozone sterilization.
- Market Opportunity: The expanding low-temperature sterilization market presents a significant opportunity for Mesa Labs to offer specialized monitoring products and services.
Technological factors are driving significant innovation in sterilization monitoring for Mesa Labs. The increasing adoption of automation and smart systems, coupled with AI and IoT integration, promises more precise quality control and predictive maintenance. For instance, the industrial automation market is projected to reach $320 billion by 2025, reflecting a broad trend towards efficiency and accuracy.
Digital transformation is also key, with a focus on continuous process verification and enhanced data integrity. This shift necessitates robust digital solutions for real-time data integration. Global spending on manufacturing execution systems and industrial IoT platforms is expected to exceed $15 billion by 2025, underscoring the industry's commitment to digital integration.
Furthermore, the growing demand for sustainable sterilization methods, such as low-temperature processes, requires new monitoring solutions. The low-temperature sterilization market was valued at approximately USD 1.5 billion in 2023 and is anticipated to grow significantly, presenting a clear opportunity for Mesa Labs to adapt and innovate its product offerings.
Legal factors
Mesa Labs operates within sectors governed by stringent regulations, demanding unwavering adherence to standards set by entities such as the FDA, the EU's Medical Device Regulation (MDR), and the UK's Medicines and Healthcare products Regulatory Agency (MHRA). For instance, the EU MDR, which fully applied from May 2021, significantly increased the scrutiny and compliance burden for medical device manufacturers, with ongoing implementation and updates impacting market access.
Mesa Labs faces significant legal hurdles concerning data integrity and privacy, especially with its increasing reliance on digital solutions for critical processes. Regulations like the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) are paramount. These laws mandate stringent measures for ensuring the accuracy, security, and confidentiality of sensitive data, particularly in healthcare and life sciences where Mesa Labs operates.
Compliance with these evolving global data protection laws requires Mesa Labs to meticulously audit its software and data management systems. Failure to adhere to these regulations can result in substantial fines; for instance, GDPR violations can lead to penalties of up to 4% of global annual revenue or €20 million, whichever is higher. Ensuring data integrity is not just a legal requirement but a fundamental aspect of maintaining customer trust and operational credibility in highly regulated industries.
The medical device and pharmaceutical industries operate under incredibly strict product liability laws, making quality assurance absolutely critical. Mesa Labs' offerings are designed to directly help their clients adhere to these demanding quality standards. This is particularly important as regulatory bodies are increasing their scrutiny on traceability and comprehensive documentation.
By ensuring robust quality assurance, Mesa Labs helps its customers minimize the significant financial and reputational risks tied to product recalls and non-compliance. For instance, in 2024, the FDA issued over 3,000 product defect warnings, highlighting the ongoing challenges companies face in maintaining product integrity and regulatory adherence.
Intellectual Property Protection
Protecting intellectual property (IP) is paramount for Mesa Labs, a company heavily reliant on its innovative quality control solutions. This includes safeguarding patents for their unique technologies, trademarks for brand recognition, and proprietary software that underpins their product offerings. Mesa Labs' commitment to R&D, evidenced by its consistent investment in new product development, makes robust IP protection vital for maintaining its competitive advantage and preventing imitation.
The company's strategy to secure its innovations directly impacts its market position. For instance, strong patent portfolios can deter competitors and allow Mesa Labs to command premium pricing for its differentiated products. In 2023, Mesa Labs continued to file new patents, reinforcing its pipeline of future technologies and ensuring ongoing protection for its existing intellectual assets.
The legal landscape surrounding IP is dynamic, requiring continuous monitoring and adaptation. Mesa Labs must navigate evolving patent laws and trademark regulations globally to ensure its innovations are adequately protected across all operating regions. Failure to do so could expose the company to infringement claims or allow competitors to leverage its technological advancements without compensation.
Key aspects of Mesa Labs' IP strategy include:
- Patent Portfolio Management: Actively pursuing and maintaining patents on new quality control technologies and methodologies.
- Trademark Enforcement: Vigilantly protecting its brand names and logos against unauthorized use in the market.
- Proprietary Software Security: Implementing measures to safeguard its unique software code and algorithms from intellectual property theft.
- Licensing and Collaboration Agreements: Strategically engaging in agreements that leverage its IP while respecting its ownership rights.
International Health Regulations Enforcement
The 2024 amendments to the International Health Regulations (IHR) are set to significantly reshape global health security. These updates aim to bolster the framework for preventing, protecting against, and controlling the international spread of diseases. For MesaLabs, this could mean a more standardized approach to reporting outbreaks and compliance with international health security measures.
Furthermore, the anticipated 2025 WHO Pandemic Agreement introduces new legal obligations and cooperative mechanisms for member states. This agreement is designed to improve pandemic preparedness and response, which could directly influence MesaLabs' international operations, particularly concerning cross-border manufacturing and the supply chain for health products. Enhanced coordination might streamline some processes but also introduce new regulatory hurdles.
The strengthened enforcement mechanisms within these new health regulations could impact MesaLabs by requiring stricter adherence to international standards for health product development and distribution. This might necessitate greater investment in compliance and regulatory affairs to navigate the evolving legal landscape effectively. The focus on global health equity also means potential new requirements for access to essential health technologies.
- IHR Amendments 2024: Focus on strengthening national capacities for disease surveillance and response, potentially impacting MesaLabs' R&D and manufacturing site compliance.
- WHO Pandemic Agreement 2025: Expected to mandate greater transparency in pathogen data sharing and equitable access to countermeasures, influencing MesaLabs' intellectual property and global supply strategies.
- Cross-border Manufacturing: Increased international coordination may lead to harmonized standards but also require rigorous validation processes for products manufactured in multiple jurisdictions.
- Access to Health Products: New legal frameworks could influence pricing and licensing agreements for MesaLabs' innovations to ensure wider global accessibility during health emergencies.
Mesa Labs must navigate complex legal frameworks governing medical devices and data privacy, including the EU MDR and HIPAA, to ensure compliance and maintain trust. Failure to adhere to these stringent regulations can result in significant financial penalties, with GDPR violations potentially costing up to 4% of global annual revenue.
The company's robust intellectual property protection strategy is crucial for maintaining its competitive edge, especially given its reliance on innovative quality control solutions. Mesa Labs' ongoing patent filings, with new applications submitted in 2023, underscore the importance of safeguarding its technological advancements against infringement.
Evolving global health regulations, such as the 2024 amendments to the International Health Regulations and the anticipated 2025 WHO Pandemic Agreement, will likely introduce new compliance requirements for Mesa Labs. These changes could impact its international operations, supply chain strategies, and the equitable distribution of its health products.
Environmental factors
Mesa Labs' dedication to corporate responsibility is evident in its Fiscal Year 2025 ESG Program Brochure, underscoring a significant focus on environmental stewardship. The company's ambition to be a 'net positive for the world' signifies a deep integration of sustainability principles across all operational facets, not solely within its product lines.
This proactive approach means Mesa Labs is actively pursuing initiatives that go beyond mere compliance, aiming to create a beneficial environmental impact. For instance, their commitment in FY2025 includes targets for reducing their carbon footprint and enhancing resource efficiency in manufacturing processes.
Mesa Labs began quantifying and disclosing its Scope 1 and 2 greenhouse gas (GHG) emissions in 2024, marking a significant step toward environmental accountability. This inaugural assessment, conducted across all its facilities, aligns with the internationally recognized GHG Protocol, underscoring a commitment to transparency in its operational impact.
By adopting the GHG Protocol, Mesa Labs is positioning itself to better understand and manage its carbon footprint, a crucial element in addressing climate change. This initiative reflects a growing trend among businesses to integrate environmental stewardship into their core strategies, responding to increasing stakeholder demand for sustainable practices.
Mesa Labs is actively pursuing sustainable manufacturing and operations. The company is implementing energy-efficient processes across its facilities, evidenced by building upgrades designed to minimize environmental impact. For instance, facility improvements aim to reduce energy consumption and waste.
The acquisition of GKE in October 2023 further bolstered Mesa Labs' commitment to eco-friendly practices. This integration included the adoption of enhanced insulation methods specifically for energy conservation, contributing to a more sustainable operational footprint.
Waste Management and Circular Economy Principles
Mesa Labs is actively pursuing waste reduction and circular economy principles through initiatives like the 'Waste-Not-Mesa' pilot program, aiming to significantly decrease hazardous and recyclable materials entering landfills. This focus aligns with broader industry trends, as companies increasingly recognize the financial and environmental benefits of sustainable waste management. For instance, the global circular economy market was projected to reach $4.5 trillion by 2030, highlighting a substantial economic opportunity in resource efficiency.
A key component of Mesa Labs' strategy involves digitizing operations to minimize paper consumption, which directly contributes to reducing its environmental footprint. This transition to online forms and digital communication is becoming standard practice, with many businesses reporting substantial cost savings and improved operational efficiency. A 2024 report indicated that companies embracing digital transformation saw an average of 15% reduction in operational costs related to paper-based processes.
- Waste Diversion: Mesa Labs' 'Waste-Not-Mesa' pilot targets diverting hazardous and recyclable waste from traditional disposal routes.
- Digital Transformation: Efforts to go paperless through online forms and digital correspondence are central to reducing the company's environmental impact.
- Circular Economy Focus: The initiatives promote a shift towards a circular economy, emphasizing resource reuse and reduced waste generation.
- Industry Alignment: These strategies are consistent with growing global investment in circular economy models, reflecting a broader business imperative for sustainability.
Adoption of Eco-Friendly Sterilization Methods
The healthcare industry is increasingly shifting towards eco-friendly sterilization methods, primarily driven by environmental regulations and a growing emphasis on sustainability. Techniques like vaporized hydrogen peroxide (VHP) and ozone sterilization are gaining traction due to their lower energy requirements compared to traditional autoclaving and their reduced emission of harmful byproducts.
Mesa Laboratories is well-positioned to capitalize on this trend by offering robust monitoring solutions for these emerging sterilization technologies. Their expertise in biological and chemical indicators is crucial for validating the efficacy of these greener methods, ensuring patient safety while aligning with the healthcare sector's broader sustainability objectives. For instance, VHP sterilization, which operates at lower temperatures, can significantly reduce energy consumption by up to 50% compared to steam sterilization.
The market for sterilization monitoring solutions is expanding, with an estimated growth rate of 6-8% annually, reflecting the increasing adoption of advanced and environmentally conscious sterilization practices globally. Mesa Labs' commitment to developing and supporting these methods directly addresses a key environmental factor influencing the medical device and healthcare industries.
- Growing demand for low-temperature sterilization: Driven by energy efficiency and reduced environmental impact.
- Mesa Labs' role in validation: Providing essential monitoring for hydrogen peroxide and ozone sterilization.
- Sustainability alignment: Supporting healthcare's commitment to greener practices.
- Market growth: Indicating increasing adoption of advanced sterilization monitoring.
Mesa Labs is actively reducing its environmental footprint by implementing energy-efficient upgrades across its facilities, aiming to lower energy consumption and waste. The acquisition of GKE in October 2023 further enhanced their sustainability efforts, particularly through improved insulation for energy conservation.
The company's 'Waste-Not-Mesa' pilot program targets diverting hazardous and recyclable materials from landfills, reflecting a broader industry trend toward resource efficiency. In 2024, Mesa Labs began quantifying and disclosing its Scope 1 and 2 greenhouse gas emissions, adhering to the GHG Protocol for increased transparency.
Mesa Labs is also embracing digitization to reduce paper consumption, with reports showing companies can achieve up to a 15% reduction in operational costs by moving away from paper-based processes.
| Initiative | Status/Target | Impact | Reference Year |
|---|---|---|---|
| GHG Emissions Disclosure | Scope 1 & 2 quantified | Increased transparency, carbon footprint management | 2024 |
| Energy Efficiency Upgrades | Ongoing facility improvements | Reduced energy consumption and waste | FY2025 |
| Waste-Not-Mesa Pilot | Piloting waste diversion | Reduced landfill waste, circular economy principles | FY2025 |
| Digital Transformation | Paperless initiatives | Reduced paper consumption, potential cost savings | 2024 Report |
PESTLE Analysis Data Sources
Our MesaLabs PESTLE Analysis is meticulously constructed using a blend of official government publications, reputable financial institutions, and leading market research firms. This ensures that every insight into political, economic, social, technological, legal, and environmental factors is grounded in verified, current data.
