KalVista Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
KalVista Bundle
Curious about the strategic engine driving KalVista's success? This Business Model Canvas breaks down their core customer segments, value propositions, and revenue streams, offering a clear view of their operational blueprint. It's a vital resource for anyone looking to understand how KalVista effectively navigates the competitive landscape of its industry.
Unlock the complete strategic blueprint of KalVista's business model. This in-depth canvas reveals how they create, deliver, and capture value, detailing everything from key partnerships to cost structures. Gain actionable insights for your own ventures.
Dive into the specifics of KalVista's market strategy with the full Business Model Canvas. This comprehensive document illuminates their customer relationships, channels, and key resources, providing a granular look at their operational framework.
See exactly how KalVista's business model functions, from its revenue streams to its cost drivers. This detailed canvas is your key to understanding their competitive advantages and growth strategies. Perfect for analysis and strategic planning.
Ready to deconstruct KalVista's path to market leadership? The full Business Model Canvas provides a holistic view of their operations, highlighting key activities and cost management. Download it to accelerate your business acumen.
Want to replicate or innovate upon KalVista's proven model? The complete Business Model Canvas offers a detailed, section-by-section breakdown, including their unique value proposition and customer segments. Get the full picture now.
Partnerships
KalVista Pharmaceuticals is strategically aligning with key partners to ensure the global commercial success of sebetralstat, their oral treatment for hereditary angioedema (HAE). These alliances are fundamental for tackling the complexities of international regulatory approvals and market penetration. By the end of 2024, KalVista aims to have secured several more such agreements, extending its reach into major pharmaceutical markets.
Recent developments highlight this focus, with KalVista having already granted commercialization rights for sebetralstat in Japan to Kaken Pharmaceutical and in Canada to Pendopharm. These partnerships are vital for navigating distinct healthcare systems and reimbursement landscapes, thereby maximizing patient access to this potentially life-changing therapy.
KalVista Pharmaceuticals actively pursues research and development collaborations to fuel scientific progress and expand its pipeline beyond hereditary angioedema (HAE). These partnerships are crucial for advancing preclinical programs, such as those focused on oral Factor XIIa inhibitors, potentially broadening their therapeutic reach into areas like thrombosis and inflammation.
By teaming up with leading research institutions and other biotechnology firms, KalVista aims to accelerate the complex and time-consuming process of drug discovery and development. Such collaborations leverage external expertise and resources, enabling the company to explore novel therapeutic targets and indications more efficiently.
KalVista actively partners with a broad network of clinical trial sites and experienced investigators globally to execute its crucial studies, including the ongoing KONFIDENT and KONFIDENT-KID trials. These collaborations are fundamental for patient recruitment and the meticulous execution of trials, ensuring both ethical standards and operational efficiency. The robust clinical data generated from these partnerships is vital for successful regulatory submissions.
Regulatory and Advisory Relationships
KalVista Pharmaceuticals places significant emphasis on its key partnerships with regulatory bodies to ensure successful drug development and market access. Establishing strong relationships with agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), the UK's Medicines and Healthcare products Regulatory Agency (MHRA), and Japan's Ministry of Health, Labour and Welfare (MHLW) is crucial for obtaining necessary approvals.
KalVista actively engages with these regulatory authorities throughout the drug lifecycle, from early development to post-market surveillance. This collaborative approach helps the company navigate complex submission requirements and rigorous review processes, thereby increasing the likelihood of their investigational therapies meeting stringent safety and efficacy standards.
- FDA Approval Pathway: KalVista's KVD001 (selexipag) is in Phase 3 development for hereditary angioedema (HAE), a critical stage requiring close FDA collaboration for potential New Drug Application (NDA) submission.
- EMA Engagement: Similar to the FDA, interactions with the EMA are vital for securing marketing authorization in the European Union, a key market for their HAE treatments.
- Global Regulatory Strategy: Navigating the distinct regulatory landscapes of agencies like MHRA and MHLW is essential for KalVista's global commercialization efforts.
Financial and Investment Partners
KalVista Pharmaceuticals actively cultivates relationships with a robust network of financial and investment partners to fuel its groundbreaking research and development pipeline. These collaborations are crucial for securing the substantial capital required to advance its therapies from discovery through to commercialization.
The company has successfully engaged in synthetic royalty financing agreements, notably with DRI Healthcare Trust. These arrangements provide upfront capital in exchange for a share of future revenue streams from specific approved products, offering a non-dilutive funding mechanism. Furthermore, KalVista has utilized equity offerings to bolster its financial position, demonstrating investor confidence in its long-term strategy and the potential of its drug candidates.
- Synthetic Royalty Financing: Agreements with entities like DRI Healthcare Trust provide upfront capital.
- Equity Offerings: Successful capital raises in public markets demonstrate investor confidence.
- Capital for R&D: These partnerships are instrumental in funding the extensive research, development, and clinical trial phases.
- Financial Stability: Securing diverse funding sources ensures the company's operational continuity and strategic execution capabilities.
KalVista's key partnerships are crucial for global commercialization and R&D advancement. These alliances facilitate regulatory approvals and market access for sebetralstat, their HAE treatment. By the close of 2024, KalVista anticipates finalizing several more strategic agreements to expand its market presence.
What is included in the product
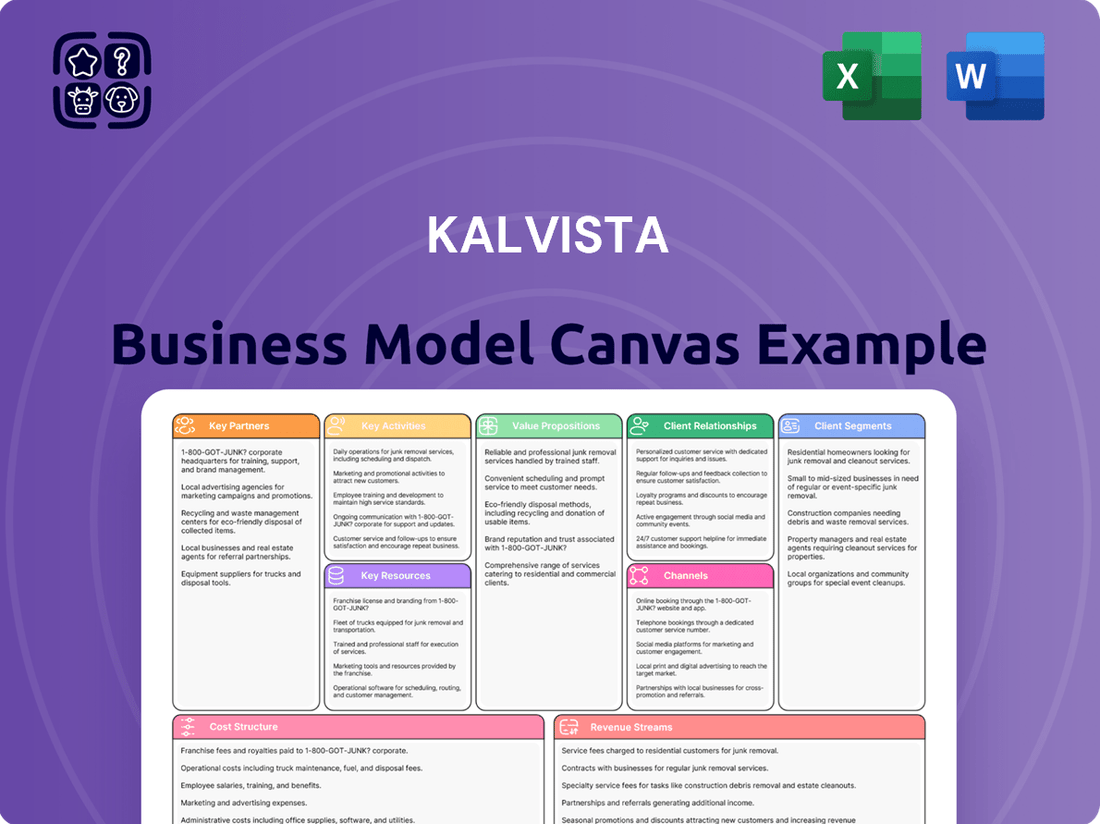
A detailed business model canvas for KalVista, outlining its strategy for developing and commercializing hereditary angioedema treatments, covering key aspects from customer segments to revenue streams.
The KalVista Business Model Canvas offers a structured approach to visualize and refine their strategy, effectively addressing the pain point of complex business planning by condensing it into a digestible format for quick review.
KalVista's Business Model Canvas serves as a powerful tool to simplify intricate strategies, providing a clear, one-page snapshot that alleviates the pain of information overload and promotes efficient understanding.
Activities
KalVista's central mission revolves around the discovery and development of innovative small molecule protease inhibitors, particularly targeting plasma kallikrein. This crucial activity encompasses in-depth scientific research, comprehensive preclinical testing, and a meticulously structured clinical trial process to bring promising drug candidates for conditions with significant unmet medical needs to fruition.
In 2024, the company continued to advance its lead programs, notably KVD001 for hereditary angioedema (HAE), with clinical data expected to guide further development. The financial investment in research and development remains substantial, reflecting the complex and lengthy nature of drug development, often spanning over a decade and costing hundreds of millions of dollars per successful drug.
The preclinical phase involves extensive laboratory and animal studies to assess safety and efficacy, a critical step before human trials. For instance, in 2024, KalVista would have been meticulously analyzing data from these studies to ensure KVD001 met stringent regulatory requirements for progression into later-stage clinical trials.
Clinical trials are conducted in phases, from Phase 1 (safety in healthy volunteers) to Phase 2 (efficacy and dosing in patients) and Phase 3 (large-scale efficacy and safety confirmation). Successfully navigating these stages is paramount for bringing a new drug to market, a process that requires immense capital and scientific expertise.
KalVista Pharmaceuticals' core operations heavily rely on the meticulous management of complex global clinical trials. This includes overseeing pivotal studies like the Phase 3 KONFIDENT trial, which is designed to assess the efficacy and safety of sebetralstat for the treatment of hereditary angioedema (HAE).
The company also actively manages ongoing pediatric and adolescent studies, such as KONFIDENT-S and KONFIDENT-KID, to broaden the application of its therapies. These efforts are crucial for gathering robust data on patient recruitment, precise data collection, and rigorous analysis.
Ensuring strict adherence to international regulatory standards, including those set by the FDA and EMA, is paramount throughout these trials. This meticulous compliance is fundamental in demonstrating the therapeutic benefits and safety profile of KalVista's investigational treatments to regulatory bodies.
KalVista's key activities prominently feature the meticulous preparation and submission of New Drug Applications (NDAs) and Marketing Authorization Applications (MAAs) to global regulatory authorities. This critical step involves navigating the complex requirements of agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), the UK Medicines and Healthcare products Regulatory Agency (MHRA), and Japan's Pharmaceuticals and Medical Devices Agency (PMDA).
Successful navigation of these regulatory pathways is paramount for KalVista to secure market approval for its innovative therapies. The company's commitment to robust data generation and clear, comprehensive submission dossiers directly impacts its ability to bring life-changing treatments to patients worldwide. In 2024, the pharmaceutical industry continued to see significant investment in regulatory affairs, with companies dedicating substantial resources to clinical trial data compilation and submission preparation.
Commercialization and Market Entry Planning
Following regulatory approvals, KalVista Pharmaceuticals actively develops robust commercialization strategies. This includes establishing a global commercial infrastructure and equipping sales teams with comprehensive product knowledge and market insights.
Market entry planning is critical, focusing on securing reimbursement and ensuring broad patient access in key geographical regions. This preparatory work is essential for a successful product launch and sustained market penetration.
- Global Commercial Organization: Building and training a dedicated sales force for effective product promotion and customer engagement.
- Market Access Strategies: Developing plans to ensure reimbursement, pricing, and distribution of therapies in target markets.
- Product Launch Planning: Executing phased launches in key territories, supported by marketing and educational initiatives.
- Pharmacoeconomic Data: Gathering and presenting data to payers to demonstrate the value and cost-effectiveness of KalVista's treatments.
Intellectual Property Management
KalVista Pharmaceuticals actively manages its intellectual property portfolio, a critical ongoing activity. This involves securing and defending patents for its innovative drug candidates, such as those targeting hereditary angioedema (HAE), and the underlying technologies. This strategy is crucial for maintaining a significant competitive edge in the biopharmaceutical market and safeguarding the substantial investments made in research and development.
The company's commitment to intellectual property protection is demonstrated by its patent filings and granted patents. For instance, KalVista has secured broad patent protection for its plasma kallikrein inhibitors, including KVD001 and KVD002, which are designed to treat HAE. These patents typically have expiration dates extending well into the future, providing a significant period of market exclusivity.
- Patent Protection: KalVista actively pursues patent protection for its novel drug candidates and delivery systems, ensuring exclusivity for its innovations.
- Competitive Advantage: Maintaining a strong IP portfolio is essential for securing a competitive advantage against rivals and preventing unauthorized use of its technologies.
- R&D Investment Security: Intellectual property rights protect the company's significant investments in research and development, ensuring a return on innovation.
- Licensing Opportunities: A robust IP portfolio can also facilitate strategic partnerships and licensing agreements, generating additional revenue streams.
KalVista's core activities center on the rigorous advancement of its drug candidates through the development pipeline. This involves managing complex global clinical trials, such as the pivotal Phase 3 KONFIDENT trial for sebetralstat in hereditary angioedema (HAE), and also includes crucial pediatric studies like KONFIDENT-S and KONFIDENT-KID.
What You See Is What You Get
Business Model Canvas
The Business Model Canvas you are previewing is the exact document you will receive upon purchase. This isn't a mockup or a sample; it's a direct representation of the comprehensive file you'll gain access to. Once your order is complete, you'll get the full, unedited version of this professionally structured Business Model Canvas, ready for your immediate use and customization.
Resources
KalVista's most valuable resource is its proprietary portfolio of small molecule protease inhibitors. These unique compounds, developed over years of dedicated research and development, offer novel therapeutic avenues for patients with significant unmet medical needs.
Sebetralstat, a key asset within this portfolio, stands out as an oral on-demand therapy designed for Hereditary Angioedema (HAE). This signifies a significant advancement in patient care, moving towards more convenient and effective treatment options.
As of early 2024, sebetralstat has successfully completed its Phase 3 trial for HAE, demonstrating strong efficacy and a favorable safety profile. This clinical success is a testament to the quality and potential of KalVista's proprietary inhibitor technology.
The company's intellectual property surrounding these inhibitors is robust, providing a strong competitive advantage. This foundation is crucial for securing partnerships and commercializing these innovative therapies in the highly competitive pharmaceutical market.
The extensive positive clinical trial data, particularly from the KONFIDENT study showcasing sebetralstat's efficacy and safety in treating hereditary angioedema (HAE), represents a cornerstone of KalVista's value proposition. This data is not just supportive; it's the bedrock for securing regulatory approvals from bodies like the FDA and EMA, which are essential for market entry.
Robust intellectual property rights, including patents covering sebetralstat's composition of matter and methods of use, provide critical commercial exclusivity. These patents, with varying expiry dates extending well into the 2030s, shield KalVista from direct competition, allowing for premium pricing and recouping significant R&D investments.
This combination of compelling clinical evidence and strong IP protection directly translates into a significant competitive advantage. It underpins the company's ability to attract investment, negotiate favorable licensing deals, and ultimately build a sustainable revenue stream for its HAE treatment.
KalVista Pharmaceuticals' scientific and medical expertise is a cornerstone of its business model, particularly in its focus on Hereditary Angioedema (HAE). The company boasts a team of seasoned scientists and medical professionals who possess profound knowledge of rare diseases and the intricate kallikrein-kinin system, which is central to HAE's pathophysiology.
This specialized human capital is the engine behind KalVista's innovation and its ability to advance drug development pipelines effectively. Their deep understanding allows for targeted research and the creation of novel therapeutic candidates designed to address unmet needs in HAE treatment.
The caliber of their scientific team directly translates into robust clinical execution. This expertise is crucial for navigating the complexities of clinical trials, from trial design and patient recruitment to data analysis and regulatory submissions, ensuring the efficient progression of their drug candidates.
For instance, KalVista's lead HAE candidate, sebetacaine, has shown promising results in clinical trials, a testament to the team's scientific acumen. As of early 2024, the company continues to advance its research, leveraging this core strength to push the boundaries of HAE therapy.
Financial Capital and Funding
KalVista's financial capital is a critical engine for its business model, powering the entire drug development lifecycle from research to market launch.
Significant financial resources are secured through various avenues, including equity offerings, synthetic royalty financing, and strategic investments. These funding mechanisms are vital for supporting the substantial costs associated with drug development, conducting extensive clinical trials, and executing global commercialization strategies.
As of April 30, 2025, KalVista reported a robust financial position, holding approximately $220.6 million in cash, cash equivalents, and marketable securities. This substantial liquidity underscores the company's ability to finance its ambitious pipeline and operational needs.
- Equity Offerings: Provides substantial capital infusion through stock sales.
- Synthetic Royalty Financing: Offers an alternative funding route, potentially easing cash flow pressures.
- Strategic Investments: Attracts capital from partners aligned with KalVista's development goals.
- Cash Reserves: The $220.6 million as of April 30, 2025, ensures operational stability and funding for ongoing R&D and clinical trials.
Global Regulatory Approvals and Designations
Global regulatory approvals are critical resources, opening doors for commercialization and establishing competitive advantages. For KalVista, obtaining Orphan Drug Designation in Japan for sebetralstat is a significant step. This designation can offer market exclusivity and other incentives, fostering a more favorable environment for the drug's launch.
Securing FDA and UK MHRA approvals for EKTERLY (sebetralstat) represents a major regulatory milestone. These approvals are not just permissions to sell; they are endorsements of the drug's safety and efficacy, paving the way for market access in key regions. The ability to commercialize EKTERLY in these major markets is a direct result of successful navigation of rigorous regulatory pathways.
- Orphan Drug Designation in Japan: Grants a period of market exclusivity and potential fee waivers, accelerating development and commercialization efforts.
- FDA Approval for EKTERLY (sebetralstat): Enables marketing and sale within the United States, a crucial market for rare disease therapies.
- UK MHRA Approval for EKTERLY (sebetralstat): Allows for commercialization in the United Kingdom, expanding market reach and revenue potential.
- Market Access and Competitive Advantage: These designations and approvals directly translate into the ability to generate revenue and position EKTERLY favorably against potential competitors.
KalVista's key resources include its proprietary small molecule protease inhibitors, with sebetralstat being a prime example targeting Hereditary Angioedema (HAE). The company's substantial cash reserves, totaling approximately $220.6 million as of April 30, 2025, are crucial for funding ongoing research, clinical trials, and commercialization efforts. Furthermore, the robust intellectual property portfolio, including patents extending into the 2030s, provides a significant competitive moat.
| Resource Category | Specific Resource | Key Value/Status |
|---|---|---|
| Proprietary Technology | Small Molecule Protease Inhibitors | Novel therapeutic avenues for unmet medical needs, including sebetralstat for HAE. |
| Financial Capital | Cash, Cash Equivalents, and Marketable Securities | $220.6 million as of April 30, 2025, ensuring operational continuity and R&D funding. |
| Intellectual Property | Patents for sebetralstat and methods of use | Commercial exclusivity extending into the 2030s, protecting against competition. |
| Human Capital | Scientific and Medical Expertise | Deep understanding of rare diseases and kallikrein-kinin system, driving drug development. |
| Regulatory Approvals | FDA and UK MHRA Approval for EKTERLY (sebetralstat) | Market access in key regions, validating safety and efficacy. |
Value Propositions
KalVista's EKTERLY (sebetralstat) stands as the sole oral, on-demand therapy for hereditary angioedema (HAE) attacks, catering to patients aged 12 and above. This distinction is crucial, as it directly addresses a significant unmet need for a more convenient and less burdensome treatment option.
The oral administration of EKTERLY offers a distinct advantage over current injectable HAE treatments. This innovation promises enhanced patient convenience, simpler self-administration, and a notable improvement in the overall quality of life for individuals managing HAE.
For patients, the shift from injections to an oral pill represents a substantial step forward in managing their condition. This improved accessibility and ease of use can lead to better adherence and a reduced impact of HAE on daily activities.
KalVista Pharmaceuticals is keenly focused on conditions where patients and healthcare providers are seeking better solutions. For instance, Hereditary Angioedema (HAE) is a prime example of a disease with significant unmet medical needs. Existing treatments can be invasive or inconvenient, leaving a clear opening for more patient-friendly options.
This is precisely where sebetralstat, KalVista's oral kallikrein inhibitor, aims to make a difference. By offering an effective, non-invasive treatment route, sebetralstat empowers individuals with HAE to take greater control over their health. The goal is to provide a more manageable and less disruptive way to address HAE attacks.
In 2024, the market for rare disease treatments continues to expand, with a strong emphasis on patient-centric innovations. Companies like KalVista are positioned to benefit by directly addressing the limitations of current therapeutic landscapes. The drive for oral, on-demand therapies in conditions like HAE reflects a broader trend towards improving quality of life for patients with chronic illnesses.
Clinical data, including from the KONFIDENT-S study, shows sebetralstat offers swift and reliable relief from HAE attack symptoms. This rapid response means patients can get back to their lives much faster.
The effectiveness of sebetralstat is consistent across various attack locations and even different severities, providing predictable relief. This is crucial for individuals facing unpredictable and potentially dangerous swelling.
In 2024, ongoing trials continue to reinforce the value of rapid symptom control for HAE patients. The ability to quickly manage an attack significantly improves a patient's quality of life and reduces the burden of the disease.
Reduced Treatment Burden
KalVista's oral formulation for HAE treatment significantly lightens the burden compared to existing injectable options. This shift away from complex or frequent injections directly translates to improved patient convenience and greater freedom for individuals managing HAE, allowing them to participate more fully in daily activities.
The ease of taking an oral medication fosters better adherence to treatment regimens. This improved compliance is crucial for managing chronic conditions like HAE effectively. For instance, in the HAE market, patient adherence is a key factor in achieving optimal health outcomes and minimizing disease impact.
This reduced treatment burden is a core value proposition because it addresses a significant unmet need. Patients often prefer less invasive and simpler treatment methods. The convenience factor can lead to a more positive patient experience and potentially better long-term management of their condition, aligning with the growing trend towards patient-centric healthcare solutions.
- Reduced Administration Complexity: Eliminates the need for trained personnel or self-injection techniques associated with parenteral drugs.
- Enhanced Patient Convenience: Allows for discreet and easy administration, fitting seamlessly into daily routines.
- Improved Treatment Adherence: Simpler regimens often lead to higher compliance rates, crucial for chronic disease management.
- Greater Lifestyle Integration: Empowers patients to live more normally without the constant logistical considerations of injectable medications.
Potential for Foundational HAE Therapy
KalVista's ambition is for sebetralstat to establish itself as the cornerstone on-demand treatment for Hereditary Angioedema (HAE) globally. This vision is built on the therapy's demonstrated safety profile, its effectiveness in clinical trials, and the significant patient convenience offered by its oral administration. The company aims for sebetralstat to be the go-to solution for managing HAE attacks, fundamentally changing how the condition is treated.
This strategic positioning as a foundational therapy reflects a deep understanding of unmet needs in HAE management. By offering a safe, effective, and easily administered oral option, KalVista anticipates sebetralstat will become the preferred choice for patients and physicians alike, potentially capturing a substantial share of the HAE market. For instance, the global HAE market was valued at approximately $2.5 billion in 2023 and is projected to grow, underscoring the significant opportunity for a foundational therapy.
- Transformative Potential: Sebetralstat's oral administration offers a significant advantage over existing injectable therapies, improving patient adherence and quality of life.
- Market Leadership: KalVista's goal is to make sebetralstat the first-line, on-demand treatment for all HAE patients worldwide.
- Safety and Efficacy: Clinical data supports sebetralstat's profile as a well-tolerated and effective treatment for acute HAE attacks.
- Market Penetration: Achieving foundational status would allow sebetralstat to address a broad spectrum of HAE patients, driving significant revenue growth.
KalVista's EKTERLY (sebetralstat) offers a unique value proposition as the sole oral, on-demand therapy for hereditary angioedema (HAE) attacks. This oral formulation significantly enhances patient convenience and adherence compared to existing injectable treatments, addressing a critical unmet need in HAE management. The drug's efficacy in providing rapid and reliable symptom relief, as demonstrated in clinical studies like KONFIDENT-S, further solidifies its position as a transformative treatment option.
Customer Relationships
KalVista Pharmaceuticals actively cultivates direct relationships with patients and their caregivers, a cornerstone of their customer relationship strategy. This engagement is crucial for deeply understanding the unique challenges faced by individuals with Hereditary Angioedema (HAE).
Through educational initiatives and dedicated patient advocacy programs, KalVista aims to empower the HAE community. For instance, their commitment is reflected in resources designed to explain complex medical information in accessible terms, fostering a more informed patient base.
This direct feedback loop is invaluable, directly informing KalVista's product development pipeline and support services. By listening to patients, they ensure that innovations and assistance are precisely targeted to enhance the quality of life for those living with HAE.
In 2024, the company continued to expand its patient support programs, with a reported increase in engagement metrics across their digital platforms. This focus on user interaction underscores their dedication to building trust and providing ongoing value beyond the initial treatment.
KalVista Pharmaceuticals prioritizes building robust partnerships with allergists, immunologists, and other specialists focused on Hereditary Angioedema (HAE). This collaborative approach is fundamental to establishing EKTERLY (sebetralstat) as a trusted treatment option.
To foster these vital connections, KalVista actively engages in providing comprehensive medical education and disseminating crucial clinical data. This ensures healthcare providers are thoroughly informed about EKTERLY's profile and its optimal application in patient care.
By offering ongoing support, KalVista empowers healthcare professionals with the knowledge and resources necessary to confidently prescribe and manage EKTERLY. This commitment to education and support is a cornerstone of their customer relationship strategy.
KalVista Pharmaceuticals actively cultivates partnerships with patient advocacy groups worldwide, a cornerstone of their customer relationship strategy. These collaborations are vital for disseminating clinical trial data at key medical and patient-focused conferences, directly engaging with the Hereditary Angioedema (HAE) community.
By working closely with these organizations, KalVista not only raises crucial awareness about HAE but also provides essential support to affected individuals and families. This fosters a sense of community and shared purpose.
Crucially, these patient advocacy partnerships ensure that the lived experiences and perspectives of those with HAE are integrated into KalVista’s drug development process. This patient-centric approach is designed to inform treatment design and facilitate better access to therapies, reflecting a commitment beyond just product sales.
Dedicated Commercial and Medical Affairs Teams
KalVista Pharmaceuticals is strategically building specialized commercial and medical affairs teams. These dedicated groups are crucial for educating healthcare professionals and fostering engagement with key opinion leaders in the hereditary angioedema (HAE), allergy, and rare disease sectors. Their primary objective is to ensure smooth and effective access to EKTERLY (sebetralstat) for patients.
These field-based experts are being equipped with in-depth knowledge of HAE and related therapeutic areas. By focusing on physician education and KOL interaction, KalVista aims to establish strong relationships within the medical community, facilitating the successful adoption and utilization of their innovative treatment.
- Expert Field Teams: KalVista is assembling specialized commercial and medical affairs teams with deep expertise in HAE, allergy, and rare diseases.
- Physician Education: These teams will focus on educating physicians about EKTERLY (sebetralstat) and its benefits.
- Key Opinion Leader Engagement: Building relationships with influential medical experts is a core responsibility.
- Facilitating Access: The teams are tasked with ensuring patients can access EKTERLY (sebetralstat).
Transparent Investor Communications
KalVista prioritizes transparent investor communications to foster trust and keep stakeholders informed. This commitment is demonstrated through regular operational updates, detailed financial results, and engagement at investor conferences.
Maintaining open dialogue ensures that financially-literate decision-makers are aware of KalVista's progress, strategic direction, and overall financial well-being. For example, in 2024, the company actively participated in multiple investor events, providing comprehensive insights into its clinical trial advancements and financial performance.
- Operational Updates: Providing timely information on clinical trial progress and regulatory milestones.
- Financial Results: Disclosing quarterly and annual financial reports that detail revenue, expenses, and cash flow.
- Investor Conferences: Engaging directly with investors to answer questions and present strategic outlooks.
- Proactive Disclosure: Ensuring all material information is communicated promptly and clearly to the market.
KalVista Pharmaceuticals cultivates multifaceted customer relationships, engaging directly with patients, caregivers, healthcare professionals, and investors. This comprehensive approach ensures deep understanding and support for the Hereditary Angioedema (HAE) community, while fostering strong partnerships within the medical field and maintaining transparent communication with stakeholders.
Channels
KalVista is building a direct sales force in crucial markets, starting with the U.S. This team will be composed of individuals with deep expertise in hereditary angioedema (HAE) and rare disease treatment.
This specialized sales force will directly interact with healthcare providers. Their primary goal is to educate physicians about EKTERLY (sebetralstat) and encourage its prescription.
The U.S. market for HAE treatments saw significant growth, with estimates suggesting the market could reach several billion dollars by 2028, driven by new therapies and increasing diagnosis rates. KalVista's direct sales approach aims to capture a substantial share of this expanding market by fostering strong relationships with key opinion leaders and prescribers.
KalVista Pharmaceuticals will leverage existing, robust pharmaceutical distribution networks to ensure EKTERLY (sebetralstat) reaches its intended destinations. This strategy is crucial for patient access, targeting pharmacies, hospitals, and specialized clinics. The company will partner with established wholesalers and distributors to manage the complex logistics and intricate supply chain required for a novel pharmaceutical product.
Working with these established channels in 2024, particularly within the US market, means navigating a landscape where major distributors like McKesson, AmerisourceBergen, and Cardinal Health hold significant market share, often exceeding 90% of pharmaceutical distribution. This existing infrastructure minimizes the need for KalVista to build its own costly distribution system, allowing for a more efficient and cost-effective rollout of EKTERLY.
KalVista Pharmaceuticals actively cultivates strategic commercial partnerships to expand its international presence and market access. These collaborations are crucial for navigating diverse regulatory landscapes and achieving effective commercialization in key global markets.
For instance, in Japan, KalVista has partnered with Kaken Pharmaceutical. This alliance allows Kaken to manage the complex process of regulatory approval and subsequent commercialization of KalVista's products within Japan, thereby leveraging Kaken's established infrastructure and local market expertise.
Similarly, in Canada, Pendopharm serves as KalVista's commercial partner. This arrangement enables Pendopharm to handle regulatory submissions and the go-to-market strategy for KalVista's offerings in the Canadian market, ensuring efficient product launch and distribution.
These partnerships are designed to accelerate global reach, allowing KalVista to focus on its core strengths in drug development while relying on local expertise for market entry and sales. This model proved effective, contributing to the company's growth trajectory leading into 2024.
Digital and Medical Marketing
KalVista leverages digital marketing extensively to reach healthcare providers, focusing on educational content about Hereditary Angioedema (HAE) and EKTERLY (sebetralstat). This includes targeted online advertising and search engine optimization to ensure key information is readily available.
The company utilizes medical marketing channels such as online medical journals, virtual symposia, and webinars to engage with physicians and specialists. These platforms are crucial for disseminating clinical data and treatment guidelines.
- Digital Platforms: KalVista maintains a strong online presence through its corporate website and dedicated patient/physician portals, offering educational resources and product information.
- Scientific Publications: Dissemination of clinical trial results and research findings in peer-reviewed medical journals is a cornerstone of their strategy.
- Virtual Congresses: Participation in virtual medical conferences allows for broad reach to healthcare professionals globally, presenting the latest data on EKTERLY.
- Professional Outreach: Direct digital outreach to medical professionals via email campaigns and professional networking sites like LinkedIn are employed to share updates and educational materials.
Patient Support Programs
Patient support programs act as a crucial channel for delivering direct assistance and education to individuals prescribed EKTERLY (sebetralstat). These initiatives are designed to empower patients by providing them with the necessary resources to manage their condition effectively. For instance, in the US, patient assistance programs for specialty medications often report high engagement rates, with some programs seeing up to 70% of eligible patients enrolling to receive support.
These programs are instrumental in helping patients navigate the complexities of accessing and adhering to their treatment. This can include assistance with insurance coverage, co-pay assistance, and educational materials on how to properly administer EKTERLY. A significant portion of patients, estimated at around 30% for many chronic conditions, require some form of support to maintain consistent medication use, highlighting the importance of these channels.
- Direct Patient Assistance: Providing financial aid for co-pays and deductibles, a common feature in many biopharmaceutical patient support offerings.
- Educational Resources: Offering information on hereditary angioedema (HAE), treatment protocols, and lifestyle management.
- Adherence Support: Implementing reminder systems and personalized coaching to ensure consistent medication intake.
- Access Navigation: Assisting patients with insurance verification, prior authorization processes, and identifying external financial assistance.
KalVista utilizes a multi-pronged channel strategy, combining direct sales with established distribution networks and strategic partnerships. This approach ensures broad market reach and efficient product delivery. For 2024, the focus remains on building a specialized US sales force for direct physician engagement, while leveraging existing pharmaceutical infrastructure for broader distribution.
International expansion is facilitated through commercial partnerships, with entities like Kaken Pharmaceutical in Japan and Pendopharm in Canada handling regulatory and market access complexities. Digital marketing and medical education channels are also key, targeting healthcare providers with vital information on EKTERLY. Patient support programs are integral for adherence and access, providing crucial assistance to individuals managing HAE.
| Channel | Strategy | Key Activities | 2024 Focus | Partners/Examples |
|---|---|---|---|---|
| Direct Sales Force | Physician Education & Prescription | Direct interaction with HAE specialists | US market penetration | Internal specialized team |
| Distribution Networks | Product Logistics & Availability | Partnering with established wholesalers | Efficient US rollout | McKesson, AmerisourceBergen, Cardinal Health |
| Commercial Partnerships | International Market Access | Regulatory approval & commercialization | Global expansion | Kaken Pharmaceutical (Japan), Pendopharm (Canada) |
| Digital Marketing | Healthcare Provider Engagement | Targeted advertising, SEO, webinars | Information dissemination | Corporate website, online journals |
| Patient Support Programs | Patient Adherence & Access | Co-pay assistance, educational resources | Empowering patients | Internal programs, potential third-party providers |
Customer Segments
KalVista's primary customer segment comprises adults diagnosed with hereditary angioedema (HAE) who suffer from acute attacks. This group represents a critical market due to the substantial unmet need for convenient, oral on-demand treatment options. As of early 2024, HAE affects an estimated 1 in 10,000 to 1 in 50,000 people globally, with a significant portion of these individuals being adults experiencing recurrent, debilitating attacks.
KalVista is targeting adolescents aged 12 and older with HAE, a significant expansion given EKTERLY's FDA approval for this group. This move offers a more convenient oral treatment alternative for a younger demographic that historically relied on injections. The market for HAE treatments is substantial, with estimates suggesting the global HAE market could reach over $3 billion by 2027, and this younger segment represents a key growth area.
KalVista is strategically targeting pediatric patients aged 2 to 11 years with Hereditary Angioedema (HAE), recognizing a significant unmet need for accessible oral on-demand therapies. The ongoing KONFIDENT-KID trial is a key initiative designed to evaluate the safety and efficacy of their investigational treatment in this specific age group.
The development of an orally disintegrating tablet (ODT) formulation is crucial for this segment, as it offers a more convenient and palatable administration method for young children, potentially improving adherence and treatment outcomes. This focus directly addresses the challenges associated with traditional administration methods in pediatric care.
As of early 2024, HAE affects an estimated 1 in 50,000 people worldwide, with a significant portion being children. The availability of an ODT formulation could dramatically improve the quality of life for these young patients, enabling them to manage HAE attacks more effectively and with less distress.
KalVista's commitment to this pediatric segment underscores a broader market trend towards developing specialized treatments for younger populations, acknowledging their unique physiological needs and the importance of early intervention in chronic conditions like HAE.
Patients Experiencing Attacks Despite Prophylaxis
This specific patient segment comprises individuals with hereditary angioedema (HAE) who, despite adhering to long-term prophylaxis (LTP), continue to experience breakthrough attacks. These breakthrough events represent a significant unmet need, as current prophylaxis strategies do not offer complete protection for all patients. Sebetralstat’s potential to provide rapid relief for these breakthrough attacks, irrespective of the specific mechanism of the patient's current LTP, positions it as a crucial on-demand treatment for this group.
For these patients, the goal is to quickly manage acute HAE attacks that occur even while on preventative therapy. The efficacy of sebetralstat in delivering swift symptom resolution is paramount.
- Unmet Need: Patients on LTP still suffer breakthrough attacks.
- Sebetralstat's Role: Offers rapid on-demand relief for these breakthrough events.
- Broad Applicability: Effective regardless of the patient's specific LTP.
- Value Proposition: Provides a critical solution for a segment with persistent symptomatic episodes.
Healthcare Professionals (Allergists, Immunologists, Rare Disease Specialists)
Healthcare professionals, specifically allergists, immunologists, and specialists in rare diseases, represent a critical customer segment for KalVista. While they may not be the direct end-users of their treatments, their role as prescribers and key influencers in the Hereditary Angioedema (HAE) market is paramount.
KalVista's strategy involves direct engagement and education to ensure these specialists are well-informed about their therapeutic offerings. This focus is essential for driving adoption and ensuring appropriate patient access to innovative HAE treatments.
- Key Influencers: Allergists and immunologists are typically the first point of contact for patients experiencing angioedema symptoms, making their diagnostic capabilities and prescribing habits vital.
- Medical Education Focus: KalVista prioritizes providing comprehensive medical education, including data from clinical trials and real-world evidence, to these specialists.
- Rare Disease Expertise: Specialists in rare diseases possess the in-depth knowledge required to manage complex conditions like HAE, understanding the nuances of patient care and treatment selection.
- Market Penetration Strategy: By targeting these specific medical communities, KalVista aims to build strong relationships and establish its treatments as a preferred option for HAE management.
KalVista's customer segments extend to payers, including insurance companies and government health programs, who determine formulary placement and reimbursement for HAE treatments. Their decisions significantly impact patient access and affordability.
The company's engagement with payers focuses on demonstrating the clinical and economic value of its therapies. Presenting robust data on efficacy, safety, and potential cost savings compared to existing treatments is crucial for securing favorable reimbursement. In 2024, the increasing focus on value-based healthcare models among payers makes this segment particularly important.
Understanding payer needs involves highlighting how KalVista's treatments can potentially reduce overall healthcare utilization and costs associated with HAE, such as emergency room visits and hospitalizations. This data-driven approach is key to gaining market access.
Cost Structure
Research and Development (R&D) is a core cost driver for KalVista Pharmaceuticals, reflecting the capital-intensive nature of drug discovery and development. These expenses encompass a broad spectrum of activities, from initial preclinical research to the extensive and often lengthy process of clinical trials. The company's commitment to innovation necessitates substantial ongoing investment in these crucial stages.
For the fiscal year concluding on April 30, 2025, KalVista reported R&D expenses totaling $71.7 million. This figure underscores the significant financial resources allocated to advancing their pipeline candidates through rigorous testing and regulatory pathways. Such expenditures are fundamental to bringing novel therapies to market and are a key component of their operational cost structure.
General and administrative (G&A) expenses represent a significant cost for KalVista, encompassing crucial activities like pre-commercial planning and the establishment of commercial and sales teams. This category also includes the ongoing corporate overhead necessary to operate the business.
For the fiscal year ending April 30, 2025, KalVista reported G&A expenses of $116.3 million. This increase was largely attributable to the intensified efforts and investments made in preparation for commercialization.
Clinical trial execution, particularly for large-scale, multinational studies like KalVista's KONFIDENT and KONFIDENT-KID, represents a significant portion of their Research and Development (R&D) expenses. These costs encompass a wide array of activities, from enrolling participants across various geographical locations to managing the numerous clinical sites involved.
The intricate logistics of managing global trials, including data collection, quality assurance, and navigating diverse regulatory landscapes, contribute heavily to the overall expenditure. For instance, in 2024, the average cost per patient in a Phase 3 clinical trial can range from $40,000 to $75,000, with complex trials often exceeding these figures.
Regulatory fees, essential for gaining approvals and ensuring compliance with health authorities worldwide, add another layer of substantial cost. These fees are critical for advancing drug candidates through the development pipeline, making them a non-negotiable R&D investment.
Sales and Marketing Expenses
As KalVista Pharmaceuticals transitions towards commercialization, its sales and marketing expenses are set to escalate significantly. This involves the substantial undertaking of building and training a specialized field sales force, a critical component for effectively reaching target healthcare providers and patients. These investments are directly tied to the upcoming launch of EKTERLY (sebetralstat), making them indispensable for a successful market entry and adoption.
The company's strategic approach includes robust market access initiatives aimed at ensuring EKTERLY is readily available and reimbursed for patients. Furthermore, considerable resources are allocated to comprehensive promotional activities designed to educate the medical community and raise awareness about the benefits of sebetralstat. For instance, in its fiscal year 2024, KalVista reported selling, general, and administrative expenses of $78.7 million, a substantial portion of which is dedicated to these commercial readiness efforts.
- Field Sales Team Development: Building a dedicated sales force to promote EKTERLY.
- Market Access Programs: Initiatives to ensure patient access and reimbursement for sebetralstat.
- Promotional Activities: Educational campaigns and marketing efforts for product launch.
- Increased SG&A: Fiscal year 2024 SG&A expenses reached $78.7 million, reflecting these investments.
Manufacturing and Supply Chain Costs
Once KalVista’s oral kallikrein inhibitor, KVD001, reaches commercialization, the manufacturing and supply chain will represent a significant component of its cost structure. This includes the expenses tied to producing the active pharmaceutical ingredient (API) and the final drug product, along with packaging materials and stringent quality control measures to ensure product integrity and patient safety.
Establishing a reliable global supply chain is also paramount, involving logistics, warehousing, and distribution networks to make KVD001 accessible to patients worldwide. These operational costs are critical for scaling production to meet market demand and will be a primary driver of the overall cost of goods sold.
- Manufacturing Costs: Expenses related to API synthesis, formulation, and final drug product production.
- Supply Chain Establishment: Costs for logistics, warehousing, and distribution networks for global reach.
- Quality Control: Investment in rigorous testing and compliance to meet regulatory standards.
- Packaging: Expenses for primary and secondary packaging materials, including labeling.
KalVista's cost structure is heavily influenced by its R&D investments, particularly the high costs associated with conducting large-scale clinical trials. As the company moves towards commercialization, sales, general, and administrative (SG&A) expenses are increasing to support market launch activities.
Manufacturing and supply chain costs will become significant once products like KVD001 are commercialized, encompassing production, quality control, and global distribution.
| Cost Category | FY Ending April 30, 2025 (Millions USD) | Key Drivers |
|---|---|---|
| Research and Development (R&D) | $71.7 | Preclinical and clinical trials, regulatory submissions |
| General and Administrative (G&A) | $116.3 | Pre-commercial planning, corporate operations |
| Selling, General & Administrative (SG&A) - Combined | $78.7 (FY 2024) | Sales force development, market access, promotion |
Revenue Streams
The primary revenue engine for KalVista Pharmaceuticals is projected to be the direct sales of EKTERLY (sebetralstat). This anticipated income stream will fuel the company's growth once regulatory approvals are secured and commercial launches commence in major territories, including the United States, Europe, the United Kingdom, and Japan. The successful market introduction of EKTERLY is expected to be the principal contributor to KalVista's future financial performance.
KalVista Pharmaceuticals secures significant revenue through licensing agreements, often involving upfront payments and the potential for future milestone payments from commercial partners. This strategy allows them to monetize their innovations while expanding their global reach.
A prime example of this revenue model is the agreement with Kaken Pharmaceutical for the Japanese market. This deal included an initial $11 million upfront payment, providing immediate capital for KalVista’s operations.
Beyond the initial cash injection, the Kaken agreement also outlines potential additional milestone payments. These payments are typically triggered by the successful achievement of specific development or commercial targets, offering further upside for KalVista.
In 2024, the company continued to leverage this approach, aiming to secure similar partnerships to fund its ongoing research and development efforts for its portfolio of intravitreal therapies.
Royalties from commercial partnerships represent a significant, ongoing revenue stream for KalVista Pharmaceuticals. Beyond initial payments and milestone achievements, the company is set to receive a percentage of net sales once its partners successfully commercialize sebetralstat in various territories. This structure ensures that KalVista benefits directly from the market success of its developed therapies.
For instance, in 2024, as sebetralstat moves closer to potential commercialization, the anticipation of these royalty payments becomes a key financial projection. If a partner achieves $100 million in net sales in a given year and KalVista holds a 10% royalty rate, this would translate to $10 million in passive income for KalVista, demonstrating the long-term value of such agreements.
Potential Future Product Sales from Pipeline Expansion
Beyond sebetralstat, KalVista Pharmaceuticals anticipates future revenue from its expanded pipeline. This includes developing oral Factor XIIa inhibitors for preventing hereditary angioedema (HAE) attacks, as well as exploring applications for thrombosis and inflammation.
The company's strategy hinges on successfully advancing these candidates through clinical trials and securing regulatory approvals. For instance, the development of a prophylactic HAE treatment could open a significant new market segment, diversifying revenue beyond on-demand therapies.
- Oral Factor XIIa Inhibitors for Prophylaxis: Targeting the prevention of HAE attacks, a potentially larger market than current on-demand treatments.
- Thrombosis and Inflammation Indications: Expanding the therapeutic reach of Factor XIIa inhibitors to address other significant unmet medical needs.
- Diversification of Revenue: Reducing reliance on sebetralstat by building a portfolio of approved products.
- Potential for Significant Market Share: Successful development could position KalVista as a leader in multiple therapeutic areas.
Strategic Financing and Investment Income
KalVista Pharmaceuticals has strategically leveraged financing and investment income to support its operations, rather than relying solely on direct product sales. This approach is crucial for a biotech company focused on long-term drug development.
The company has notably utilized synthetic royalty financing and equity offerings. For instance, in 2023, KalVista announced a royalty financing agreement to advance its KVD824 program, providing significant capital without diluting equity substantially. This demonstrates a commitment to securing funding through non-dilutive or less dilutive means when possible.
Beyond these financing activities, KalVista also generates income from its cash reserves. As of December 31, 2023, the company reported cash, cash equivalents, and marketable securities totaling approximately $116.6 million. Interest earned on these holdings contributes to the overall financial resources available for research and development.
- Synthetic Royalty Financing: Provides capital without immediate equity dilution, supporting specific development programs.
- Equity Offerings: Used to raise substantial capital for broader operational needs and pipeline advancement.
- Interest Income: Generated from a robust cash and marketable securities balance, contributing to financial flexibility.
- Strategic Capital Management: Focuses on maintaining a strong cash position to navigate the lengthy drug development cycle.
KalVista Pharmaceuticals' revenue streams are multifaceted, reflecting a strategic approach to funding its drug development pipeline. The core anticipated revenue comes from the direct sales of its lead product, EKTERLY (sebetralstat), once regulatory approvals are obtained in key markets like the US and Europe.
Licensing agreements provide crucial upfront payments and future milestone revenues, as seen with the Kaken Pharmaceutical deal for Japan, which included an $11 million upfront payment in 2023. Royalties from these partnerships will also contribute significantly, with potential for substantial passive income as commercialization progresses.
The company is also developing oral Factor XIIa inhibitors for hereditary angioedema (HAE) prophylaxis and other indications, which represent future revenue diversification. Beyond product-related income, KalVista generates interest income from its substantial cash reserves, which stood at approximately $116.6 million as of December 31, 2023, and utilizes financing strategies like synthetic royalty financing to support development.
| Revenue Stream | Description | Key Example/Data Point |
| Product Sales | Direct sales of approved therapies like EKTERLY. | Anticipated in US, Europe, UK, Japan. |
| Licensing & Milestones | Upfront payments and milestone triggers from commercial partners. | Kaken Pharmaceutical deal: $11M upfront payment (2023). |
| Royalties | Percentage of net sales from commercialized products. | Potential $10M annually on $100M net sales at 10% royalty. |
| Pipeline Development | Future revenue from new drug candidates (e.g., oral Factor XIIa inhibitors). | Targeting HAE prophylaxis and thrombosis. |
| Investment Income | Interest earned on cash and marketable securities. | ~$116.6M cash, cash equivalents, and marketable securities (Dec 31, 2023). |
Business Model Canvas Data Sources
The KalVista Business Model Canvas is informed by a blend of clinical trial data, patent filings, and financial projections. This comprehensive approach ensures each component is validated by scientific evidence and market potential.
