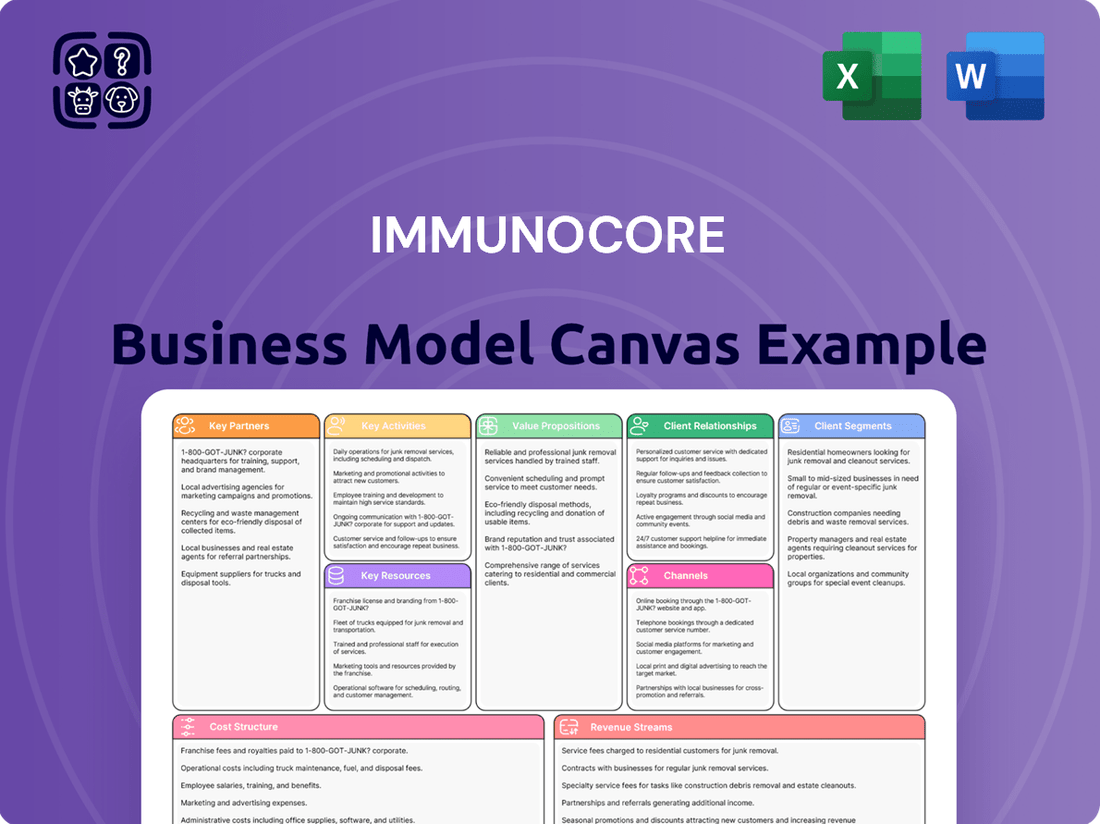
Immunocore Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Immunocore Bundle
Explore the core components of Immunocore's innovative approach with our detailed Business Model Canvas. Understand their unique value proposition, customer relationships, and key resources that drive their success in the biopharmaceutical sector. This comprehensive analysis is your key to unlocking their strategic framework.
Ready to dissect Immunocore's winning strategy? Our full Business Model Canvas provides an in-depth look at their revenue streams, cost structure, and channels. Download it now to gain actionable insights for your own business ventures.
Partnerships
Immunocore actively collaborates with top-tier academic and research institutions. These partnerships are vital for groundbreaking early-stage research, pinpointing new therapeutic targets, and gaining a deeper understanding of complex disease pathways.
By teaming up with these institutions, Immunocore taps into advanced scientific knowledge and utilizes specialized research facilities. This synergy significantly speeds up the discovery and preclinical development of novel ImmTAC molecules, aimed at treating a wide range of diseases.
Immunocore’s reliance on Clinical Research Organizations (CROs) is critical for the successful execution of its global clinical development programs. These partnerships are vital for navigating the complexities of designing, conducting, and managing trials efficiently across diverse regions.
CROs offer specialized expertise in crucial areas such as regulatory affairs, ensuring compliance with varying international standards, and patient recruitment, a key challenge in rare disease and oncology trials. Their capabilities in data management and statistical analysis are essential for generating robust evidence to support regulatory submissions, as seen with the development of ImmTACs like KIMMTRAK.
By outsourcing these functions, Immunocore can focus on its core competencies in drug discovery and development. The global CRO market size was estimated to be over $50 billion in 2023, highlighting the significant investment companies like Immunocore make in these specialized services to accelerate their pipeline progression.
Immunocore actively pursues strategic alliances with major biopharmaceutical firms to co-develop and license its ImmTAC platform. These collaborations are crucial for securing substantial funding, mitigating development risks, and leveraging partners' extensive commercialization expertise.
For instance, in April 2024, Immunocore announced an expansion of its existing collaboration with Genmab, focusing on the development of novel bispecific antibodies targeting specific cancer indications. This partnership aims to accelerate the clinical progression of ImmTAC candidates and broaden their therapeutic applications.
Manufacturing and Supply Chain Partners
Immunocore’s manufacturing and supply chain partners are essential for delivering its innovative ImmTAC molecules. These collaborations ensure the consistent production of high-quality therapies and their availability to patients worldwide. For instance, in 2024, the company continued to rely on specialized contract manufacturing organizations (CMOs) for both drug substance and drug product, a common strategy in the biopharmaceutical industry to manage complex production processes and scale.
These vital partnerships encompass critical functions beyond just production. They include rigorous quality control measures to meet stringent regulatory standards, efficient logistics for timely delivery, and robust distribution networks to reach global markets. The successful commercialization of KIMMTRAK, Immunocore’s first approved therapy, and the anticipated launches of its pipeline candidates are heavily dependent on the reliability and expertise of these manufacturing and supply chain allies.
- Drug Substance Manufacturing: Partnerships with specialized facilities capable of complex biopharmaceutical synthesis.
- Drug Product Manufacturing: Collaborations for formulation, filling, and finishing of the final therapeutic product.
- Quality Control and Assurance: Agreements with accredited laboratories to ensure product safety, efficacy, and compliance.
- Logistics and Distribution: Networks for cold chain management and global supply chain operations.
Patient Advocacy Groups and Foundations
Immunocore actively collaborates with patient advocacy groups and disease-specific foundations. These partnerships are crucial for gaining a deep understanding of patient needs, amplifying disease awareness, and ensuring patient access to therapies such as KIMMTRAK. For instance, by working with groups focused on uveal melanoma, Immunocore can better tailor its support programs.
These collaborations also play a vital role in bolstering clinical trial recruitment efforts. By engaging with patient communities, Immunocore can streamline the process of identifying and enrolling eligible participants. This was evident in the recruitment for the Phase 3 IMCgp100-202 trial, which led to the approval of KIMMTRAK.
Furthermore, these partnerships provide invaluable insights into the lived experiences of patients. This feedback directly informs the development of patient-centric support programs, enhancing the overall patient journey. In 2024, the company continued to strengthen these relationships, recognizing their impact on both clinical development and post-market support.
- Patient Needs Understanding
- Disease Awareness and Access Facilitation
- Clinical Trial Recruitment Support
- Patient Experience Insights for Support Programs
Immunocore’s key partnerships extend to contract manufacturing organizations (CMOs) and specialized logistics providers. These collaborations are essential for the reliable production and global distribution of its ImmTAC therapies, including KIMMTRAK. The company relies on these partners for complex biopharmaceutical synthesis, formulation, and adherence to stringent quality control standards, ensuring patient access to critical treatments.
The company also engages with major biopharmaceutical firms through strategic alliances for co-development and licensing. These partnerships, such as the expanded collaboration with Genmab announced in April 2024, provide significant funding, share development risks, and leverage extensive commercialization expertise, accelerating the progression of ImmTAC candidates.
Crucially, Immunocore collaborates with top-tier academic and research institutions to drive early-stage discovery and target identification. These academic ties, alongside partnerships with Clinical Research Organizations (CROs), are vital for navigating complex clinical trials and generating robust data, with the global CRO market exceeding $50 billion in 2023.
| Partner Type | Purpose | Example/Data Point |
|---|---|---|
| Academic & Research Institutions | Early-stage research, target identification | Access to advanced scientific knowledge and facilities |
| Clinical Research Organizations (CROs) | Clinical trial execution, regulatory compliance | Global CRO market over $50 billion (2023) |
| Biopharmaceutical Firms | Co-development, licensing, commercialization | Expanded collaboration with Genmab (April 2024) |
| Contract Manufacturing Organizations (CMOs) & Logistics | Therapy production, global distribution | Ensuring consistent quality and patient access |
What is included in the product
This Immunocore Business Model Canvas provides a strategic overview of their TCR immunotherapy platform, detailing customer segments (patients, healthcare providers), value propositions (novel cancer treatments), and key partnerships.
It outlines revenue streams from drug sales and collaborations, cost structure focused on R&D, and key resources like intellectual property and scientific expertise.
Immunocore's Business Model Canvas acts as a pain point reliever by providing a clear, one-page snapshot of their innovative approach to developing T-cell receptor (TCR) therapies.
This concise framework helps stakeholders quickly grasp the core components of their value proposition and customer segments, alleviating the pain of understanding complex, cutting-edge biotechnology.
Activities
Immunocore's central focus is the discovery and development of innovative T cell receptor (TCR) bispecific immunotherapies, known as ImmTACs. This rigorous process includes extensive preclinical research, validating potential targets, and optimizing lead candidates to address a range of cancers, infectious diseases, and autoimmune disorders, thereby consistently growing their unique platform.
The company's commitment to innovation is reflected in its financial investments. In 2024, Immunocore allocated $222.2 million to research and development. This investment continued into the first quarter of 2025, with $56.5 million dedicated to R&D, a slight decrease from the $57.5 million spent in the first quarter of 2024, underscoring their ongoing pursuit of novel therapeutic solutions.
Immunocore's core activity revolves around the meticulous management and execution of clinical trials for its innovative ImmTAC therapies. This encompasses the entire lifecycle from initial design and patient recruitment to ongoing monitoring and data analysis across all trial phases.
A significant focus is placed on ensuring the highest standards of data integrity and patient safety throughout the trials. This rigorous oversight is crucial for meeting the stringent requirements of regulatory bodies and achieving key clinical milestones necessary for drug approvals.
As of mid-2024, Immunocore is actively enrolling patients in three pivotal Phase 3 trials for its melanoma treatments. Concurrently, the company is progressing with Phase 1/2 trials for other promising oncology indications and exploring its pipeline for infectious diseases, demonstrating a broad commitment to advancing its therapeutic candidates.
Manufacturing ImmTAC therapeutics, like KIMMTRAK, involves intricate steps from cell line development to final formulation. This ensures each batch meets stringent quality and consistency benchmarks required by global health authorities.
In 2024, Immunocore continued to optimize its manufacturing processes, investing in advanced technologies to scale production. This focus is critical for meeting the growing demand for their innovative treatments and maintaining compliance with evolving regulatory landscapes.
Regulatory Affairs and Market Access
Navigating the complex global regulatory environment is a core activity for Immunocore. This involves meticulously preparing and submitting extensive regulatory dossiers to health authorities worldwide. The company actively engages with these agencies to secure and maintain approvals for its innovative therapies.
A crucial aspect of this is developing tailored market access and reimbursement strategies for each country. This ensures that patients who can benefit from Immunocore's treatments can actually receive them. As of March 31, 2025, KIMMTRAK has achieved approval in 39 countries and has been successfully launched in 26 markets globally, demonstrating significant progress in this area.
- Global Regulatory Navigation: Securing and maintaining approvals for therapies like KIMMTRAK across diverse international health authorities.
- Dossier Preparation and Submission: Compiling and submitting comprehensive documentation to regulatory bodies.
- Health Authority Engagement: Proactive communication and collaboration with agencies like the FDA and EMA.
- Market Access and Reimbursement Strategy: Developing country-specific plans to ensure patient access and favorable reimbursement terms post-approval.
Commercialization and Sales of Approved Products
Key activities for commercialization and sales of approved products, such as KIMMTRAK, focus on building and managing effective sales teams, executing targeted marketing and promotional campaigns, and establishing robust distribution channels to ensure access for healthcare providers and patients. This involves implementing strategic approaches for market penetration, driving product adoption, and expanding global reach to optimize revenue generation.
- Sales Force Establishment: Developing and deploying specialized sales forces to engage with oncologists and other relevant medical professionals.
- Marketing and Promotion: Implementing comprehensive marketing strategies, including medical education, digital outreach, and conference participation, to build awareness and drive demand for KIMMTRAK.
- Distribution Networks: Establishing efficient and reliable distribution channels to ensure timely and consistent availability of the product to patients.
- Market Penetration and Expansion: Focusing on strategies to increase market share and expand geographical presence to maximize revenue potential, as evidenced by KIMMTRAK's full-year 2024 net sales of $310.0 million and Q1 2025 net sales of $93.9 million.
Immunocore’s key activities center on the development and commercialization of its ImmTAC platform. This involves rigorous R&D, including extensive preclinical work and clinical trial management, exemplified by their investment of $222.2 million in R&D during 2024. The company also focuses on manufacturing its therapies to high standards and navigating complex global regulatory pathways to secure approvals and market access.
| Activity | Description | Key Metrics/Data |
|---|---|---|
| Research & Development | Discovery and optimization of ImmTAC therapies. | $222.2M R&D spend in 2024; $56.5M in Q1 2025. |
| Clinical Trials | Management of trials from design to data analysis. | Phase 3 trials for melanoma ongoing; Phase 1/2 for other indications. |
| Manufacturing | Production of ImmTACs ensuring quality and consistency. | Optimization of processes for scaled production in 2024. |
| Regulatory Affairs | Securing and maintaining global drug approvals. | KIMMTRAK approved in 39 countries as of March 31, 2025. |
| Commercialization | Sales, marketing, and distribution of approved therapies. | KIMMTRAK net sales: $310.0M (FY 2024), $93.9M (Q1 2025). |
Delivered as Displayed
Business Model Canvas
The Immunocore Business Model Canvas preview you are viewing is precisely the document you will receive upon purchase. This means you're seeing the actual, complete file, not a sample or mockup. Once your order is processed, you will gain full access to this same comprehensive Business Model Canvas, ready for your immediate use and analysis.
Resources
Immunocore's ImmTAC technology platform is the cornerstone of its business, enabling the creation of novel bispecific T cell receptor immunotherapies. This unique platform allows for the targeting of intracellular cancer antigens, a significant advancement in cancer treatment.
The ImmTAC platform is the core intellectual property driving all of Immunocore's current and future drug candidates. This proprietary technology represents a distinct and powerful approach to harnessing the immune system to fight cancer.
Immunocore's intellectual property portfolio, featuring patents on their ImmTAC molecules, manufacturing methods, and therapeutic uses, forms a cornerstone of their business model. This extensive IP protection is critical for safeguarding their groundbreaking innovations and establishing a significant competitive edge in the biopharmaceutical landscape.
These patents are instrumental in attracting vital investment capital and securing market exclusivity for both their commercially available therapies and those in development. For instance, the company's foundational patents for its lead asset, KIMMTRAK (tebentafusp-gp100), are crucial for its commercial success and future pipeline development.
Immunocore's success hinges on its highly specialized scientific researchers, clinical development experts, and medical professionals. This human capital is the engine driving innovation and the successful execution of their therapeutic strategies.
Their deep expertise spans critical areas like immunology, oncology, and infectious diseases. This specialized knowledge is paramount for advancing drug development pipelines and ensuring the efficacy and safety of their novel treatments.
In 2024, Immunocore continued to invest heavily in attracting and retaining top-tier talent. The company reported employing over 500 individuals, with a significant portion holding advanced scientific degrees, underscoring the importance of this key resource.
Manufacturing Facilities and Supply Chain Infrastructure
Immunocore’s access to or ownership of specialized manufacturing facilities is a critical resource. These facilities are designed for the complex production of biologic therapies, specifically ImmTACs, ensuring high quality and consistency. This capability is vital for meeting the growing global demand for KIMMTRAK.
A robust supply chain infrastructure complements the manufacturing capabilities. This ensures timely and efficient delivery of KIMMTRAK and other pipeline assets to patients worldwide. The company’s investment in these areas directly supports its ability to scale production and meet market needs effectively.
- Manufacturing Capabilities: Specialized facilities for complex biologic therapies like ImmTACs.
- Supply Chain: Robust infrastructure for global distribution and timely delivery.
- Scalability: Ability to increase production to meet growing demand for KIMMTRAK and pipeline assets.
Financial Capital and Funding
Immunocore's financial capital is a critical resource, underpinning its ambitious research and development pipeline, extensive clinical trials, and eventual commercialization of its innovative therapies. This substantial financial backing is essential for navigating the lengthy and costly process of drug development.
As of March 31, 2025, Immunocore reported robust liquidity, holding $837.0 million in cash, cash equivalents, and marketable securities. This healthy cash position provides significant runway for ongoing operations and strategic investments.
- Cash Reserves: Maintaining substantial cash and equivalents is paramount for funding day-to-day operations and unexpected expenses.
- Access to Funding: Immunocore can leverage equity offerings or strategic collaborations to secure additional capital as needed for its growth initiatives.
- Investment in R&D: The company's financial strength directly supports its commitment to pioneering research in T-cell receptor (TCR) therapies.
- Clinical Trial Support: Significant financial resources are allocated to conducting rigorous and comprehensive clinical trials to validate the safety and efficacy of its drug candidates.
Immunocore's key resources are its proprietary ImmTAC technology, a strong intellectual property portfolio, skilled human capital, specialized manufacturing capabilities, and substantial financial resources. These elements collectively enable the company to develop and commercialize its innovative T-cell receptor therapies.
| Resource Category | Specific Resource | Significance |
|---|---|---|
| Technology Platform | ImmTAC technology | Enables targeting of intracellular cancer antigens, forming the basis of all drug candidates. |
| Intellectual Property | Patents on ImmTAC molecules, manufacturing, and therapeutic uses | Safeguards innovation, provides competitive edge, and secures market exclusivity. |
| Human Capital | Scientific researchers, clinical development experts, medical professionals | Drives innovation and execution of therapeutic strategies; over 500 employees in 2024, many with advanced degrees. |
| Physical Resources | Specialized manufacturing facilities, robust supply chain | Ensures high-quality production of ImmTACs and efficient global delivery of therapies like KIMMTRAK. |
| Financial Resources | Cash, cash equivalents, marketable securities | Funds R&D, clinical trials, and commercialization; $837.0 million in cash as of March 31, 2025. |
Value Propositions
Immunocore's ImmTAC molecules represent a groundbreaking approach to cancer treatment, designed to harness the body's own immune system. These molecules are engineered to target intracellular antigens, a capability that many other cancer therapies lack. This allows them to direct T cells, a type of immune cell, to specifically identify and destroy cancer cells, even those hiding within the body.
This novel mechanism offers a significant advantage for patients battling aggressive or treatment-resistant cancers. For instance, in uveal melanoma, a rare and often deadly form of eye cancer, Immunocore's therapy has demonstrated remarkable efficacy. In clinical trials, their lead ImmTAC, tebentafusp, showed a significant improvement in overall survival for patients with unresectable uveal melanoma compared to historical controls, with a 22% reduction in the risk of death reported.
This differentiated therapeutic strategy addresses a critical unmet need, providing a new avenue for patients who have exhausted other treatment options. The ability to target intracellular antigens opens up possibilities for treating a broader range of cancers than therapies focused solely on cell surface targets.
KIMMTRAK, Immunocore's flagship therapy, offers a crucial value proposition by significantly improving overall survival for adult patients battling unresectable or metastatic uveal melanoma. This provides a life-extending option for a group facing a severe unmet medical need, positioning KIMMTRAK as a recognized standard of care in numerous global markets.
In clinical trials, KIMMTRAK demonstrated a notable reduction in the risk of death. For instance, the Phase 3 IMMUNOCULIN-010 study showed a 49% reduction in the risk of death for patients treated with KIMMTRAK compared to investigator's choice chemotherapy. This translates to a median overall survival of 16.8 months for KIMMTRAK versus 9.7 months for chemotherapy, underscoring its life-altering impact.
Immunocore's innovative ImmTAC platform isn't limited to uveal melanoma; it's actively being developed for a wide array of other solid tumors, offering hope for patients with limited treatment options. The company is also exploring its potential in infectious diseases, including HIV and Hepatitis B, as well as autoimmune conditions, demonstrating a commitment to tackling diverse and challenging health issues.
This expansive pipeline signifies Immunocore's ambition to create transformative medicines across multiple therapeutic areas, addressing significant unmet medical needs. For instance, their work in autoimmune diseases highlights the platform's versatility, potentially offering new avenues for treatment beyond oncology.
Precision Targeting and Potent Efficacy
ImmTAC molecules are meticulously designed to zero in on cells marked by specific disease markers. This extreme precision means they can direct T cells to attack diseased cells effectively while largely leaving healthy cells untouched. This targeted approach is key to achieving strong therapeutic results with fewer unwanted side effects.
The benefit of this precision targeting translates directly into potent efficacy. By concentrating the immune response precisely where it's needed, ImmTACs aim to deliver a powerful punch against disease. This enhanced effectiveness, coupled with a potentially better safety profile, sets them apart from therapies that might have a broader, less focused impact.
- High Specificity: ImmTACs bind to unique disease antigens on cancer cells, ensuring T cells are activated only against the intended targets.
- Maximized T Cell Redirection: These molecules efficiently bring T cells into close proximity with cancer cells, facilitating their destruction.
- Reduced Off-Target Effects: The precise targeting minimizes damage to healthy tissues, contributing to an improved safety profile.
- Potential for Enhanced Efficacy: By concentrating the immune attack, ImmTACs offer the promise of greater therapeutic success in treating various cancers.
Addressing Unmet Medical Needs
Immunocore is dedicated to tackling diseases where current treatments fall short, offering significant hope to patients. Their innovative approach targets conditions with high unmet medical needs, meaning few or no effective options exist.
By developing groundbreaking therapies, such as KIMMTRAK for advanced uveal melanoma, Immunocore provides a crucial lifeline. This therapy represents a significant advancement, as it was the first approved treatment for this specific cancer in over 30 years, addressing a critical gap in patient care.
- First-in-class therapies: Immunocore pioneers novel treatment modalities for diseases with limited therapeutic options.
- Addressing severe conditions: Focus on diseases like advanced uveal melanoma, where patient outcomes are often poor with existing treatments.
- Patient impact: KIMMTRAK's approval in 2022 marked a major milestone, offering a new standard of care for a previously underserved patient population.
- Pipeline expansion: Continued research into other oncology and autoimmune indications aims to broaden the reach of their unmet medical need solutions.
Immunocore's ImmTAC technology offers a highly specific way to target diseased cells, directing the body's own T cells to attack them while minimizing harm to healthy ones. This precision leads to improved treatment effectiveness and a better safety profile, addressing significant unmet medical needs across various conditions.
The company's flagship therapy, KIMMTRAK, has demonstrated a substantial improvement in overall survival for patients with unresectable uveal melanoma, a rare and aggressive cancer. This groundbreaking treatment provides a vital new option for a patient population with limited alternatives.
Beyond uveal melanoma, Immunocore is expanding its ImmTAC platform to target a broad range of solid tumors, infectious diseases like HIV, and autoimmune conditions, showcasing the technology's versatility and potential to transform multiple therapeutic areas.
| Therapeutic Area | Key Indication | KIMMTRAK (Tebentafusp) 2024 Data Highlight |
|---|---|---|
| Oncology | Uveal Melanoma | Continued global adoption as a standard of care, with ongoing studies exploring earlier lines of therapy. |
| Oncology | Other Solid Tumors | Advancing clinical trials in indications like non-small cell lung cancer and melanoma, targeting specific tumor antigens. |
| Infectious Diseases | HIV | Early-stage research evaluating ImmTACs for potential functional cures. |
| Autoimmune Diseases | Various | Exploring ImmTACs for conditions like lupus and rheumatoid arthritis, leveraging the platform's immune modulation capabilities. |
Customer Relationships
Immunocore cultivates direct relationships with oncologists, specialists, and other key healthcare professionals. This is achieved through their dedicated medical science liaisons and sales representatives, who are crucial for disseminating vital information.
These interactions focus on educating HCPs about KIMMTRAK's clinical profile, guiding them on appropriate patient selection, and detailing its administration. Such direct engagement is fundamental in building trust and encouraging the adoption of Immunocore's innovative therapies.
In 2024, Immunocore continued its focus on these relationships, with sales representatives actively engaging with thousands of HCPs across major oncology centers. Educational programs, including symposia and advisory boards, reached over 5,000 healthcare professionals, highlighting the company's commitment to direct communication and knowledge sharing.
Immunocore prioritizes patient well-being through dedicated support, exemplified by KIMMTRAK CONNECT®. This program offers crucial financial aid, appointment coordination, and educational materials to patients and their caregivers.
These patient-centric services are designed to streamline the treatment journey, enhancing access and adherence to therapies. For instance, in 2023, KIMMTRAK CONNECT® successfully assisted thousands of patients, demonstrating a significant commitment to improving the overall patient experience.
Immunocore actively collaborates with prominent Key Opinion Leaders (KOLs) in oncology, infectious diseases, and immunology. These collaborations are fundamental to advancing clinical practice and effectively communicating scientific findings. For instance, in 2024, Immunocore continued to engage KOLs for advisory boards and speaking engagements, leveraging their expertise to shape the understanding of T-cell receptor (TCR) therapies.
KOLs are instrumental in educating the wider medical community about novel treatment modalities, such as those developed by Immunocore. Their influence extends to treatment guideline development, which directly impacts the market adoption of innovative therapies. This engagement ensures that the scientific and clinical value of Immunocore's pipeline, including its established therapies like KIMMTRAK, is recognized and integrated into standard patient care.
Regulatory and Government Stakeholder Engagement
Maintaining robust relationships with regulatory bodies like the FDA and EMA is paramount for Immunocore. These connections facilitate smoother drug approval processes and market access, directly impacting commercial viability. For instance, in 2024, successful navigation of regulatory pathways remains a critical determinant of a biopharmaceutical company's valuation and investor confidence.
Ongoing dialogue with government agencies ensures Immunocore's strategic development aligns with public health goals and evolving healthcare policies. This proactive engagement helps in anticipating and addressing potential hurdles in market access and reimbursement negotiations, which are crucial for revenue generation.
- Regulatory Approvals: Timely approvals from agencies like the FDA are essential for commercial launch and revenue realization.
- Market Access: Collaboration with government health bodies influences pricing and reimbursement strategies, critical for profitability.
- Policy Alignment: Engaging with policymakers ensures Immunocore's pipeline and strategies are aligned with national health priorities.
- Data Transparency: Open communication regarding clinical trial data and safety profiles builds trust with regulatory stakeholders.
Investor Relations and Communication
Immunocore maintains robust investor relations through consistent communication channels. This includes timely delivery of financial reports, participation in earnings calls, and engagement at investor conferences. For instance, in their 2024 reports, the company highlighted significant progress in their clinical trial programs, aiming to bolster investor confidence.
These efforts are crucial for building and sustaining investor confidence. By providing regular updates on pipeline advancements and commercial performance, Immunocore ensures its financial stakeholders are well-informed about the company's trajectory and its ability to achieve long-term growth objectives.
- Transparent Communication: Regular financial reports and earnings calls keep investors informed.
- Pipeline Updates: Information on clinical trial progress and commercial performance is shared.
- Investor Confidence: Consistent dialogue fosters trust and belief in the company's strategy.
- Financial Support: Clear communication helps secure sustained financial backing for growth.
Immunocore's customer relationships extend beyond healthcare professionals to encompass patient support programs like KIMMTRAK CONNECT®. This initiative provides vital assistance with financial aid, appointment scheduling, and educational resources for patients and their families, aiming to ease the treatment journey and improve adherence.
The company also actively engages with Key Opinion Leaders (KOLs) in relevant medical fields. These collaborations are key for disseminating scientific findings and influencing treatment guidelines, thereby enhancing the adoption of Immunocore's therapies.
Maintaining strong ties with regulatory bodies such as the FDA and EMA is critical for Immunocore's market access and commercial success. These relationships facilitate smoother approval processes and ensure alignment with public health objectives.
Channels
Immunocore's KIMMTRAK, a specialized oncology therapy, relies on a curated distribution channel. This involves a select group of specialty pharmacies and direct engagement with hospitals and treatment centers. This focused approach is crucial for managing a complex infusion therapy.
The controlled distribution ensures that KIMMTRAK is handled, stored, and administered correctly. This meticulous process is vital for patient safety and therapeutic efficacy, targeting a specific patient demographic requiring advanced cancer treatment. For instance, in 2023, KIMMTRAK generated $175.8 million in revenue, highlighting the demand within this specialized market.
Immunocore leverages a direct sales force, notably in the crucial US and European markets, to foster direct engagement with healthcare providers and institutions. This strategy is vital for educating physicians and treatment teams about KIMMTRAK, building essential relationships, and ensuring effective promotion.
Immunocore leverages major medical conferences and scientific publications as key channels to share its groundbreaking research. For instance, presenting data from their IMC-C103 program at the 2024 ASCO Annual Meeting provided critical insights into its efficacy for advanced cutaneous T-cell lymphoma, directly influencing specialist understanding and potential treatment pathways.
These platforms are vital for building Immunocore's scientific credibility. Publication of Phase 1/2 trial results for their lead asset, tebentafusp, in leading journals like The New England Journal of Medicine in 2021, underscored the clinical validation of their ImmTAC technology and informed physician adoption.
Disseminating clinical trial data through peer-reviewed journals and conference presentations allows Immunocore to reach a global audience of oncologists and researchers. This direct communication of scientific evidence is instrumental in shaping treatment guidelines and fostering the adoption of their innovative therapies in clinical practice.
Company Website and Digital Platforms
Immunocore's corporate website and digital platforms are crucial for engaging with investors, healthcare professionals, and the public. These channels offer detailed insights into the company's cutting-edge ImmTAC technology, its clinical pipeline, and approved therapies like Kimmtrak. In 2024, the company continued to update its investor relations section with quarterly earnings reports and SEC filings, ensuring transparency.
These digital assets function as a central information hub, disseminating news, clinical trial updates, and scientific publications. For instance, the website provides access to presentations from key opinion leaders and scientific congresses, reinforcing Immunocore's commitment to scientific advancement. The company also utilizes social media channels to share company milestones and engage with a broader audience.
- Website as Investor Relations Hub: Provides access to financial reports, presentations, and corporate governance information.
- Digital Platforms for HCPs: Offers scientific data, treatment guidelines, and resources for healthcare professionals.
- Public Engagement: Shares company news, patient stories, and information about their therapeutic areas.
- Transparency in 2024: Continued regular updates on pipeline progress and financial performance through digital channels.
Partnerships and Licensing Agreements
Immunocore actively pursues partnerships and licensing agreements to broaden KIMMTRAK's global reach and enter new markets. These strategic alliances are crucial for expanding patient access in territories where Immunocore lacks its own commercial infrastructure.
By collaborating with established pharmaceutical entities, Immunocore gains access to their robust distribution networks and sales forces. This allows for more efficient market penetration and patient support.
For example, in 2024, Immunocore continued to build upon its existing agreements and explore new opportunities to extend KIMMTRAK's availability. The company's strategy emphasizes leveraging the expertise and infrastructure of its partners to overcome geographical barriers and regulatory complexities.
Key aspects of these collaborations include:
- Territorial Expansion: Enabling market entry into regions where Immunocore does not have a direct operational presence.
- Commercial Infrastructure Access: Utilizing partners' established sales, marketing, and distribution capabilities.
- Risk Sharing: Often involving shared development, manufacturing, and commercialization responsibilities.
- Revenue Streams: Generating income through upfront payments, milestone achievements, and royalties on sales.
Immunocore's channel strategy for KIMMTRAK centers on specialty pharmacies and direct engagement with treatment centers to ensure proper handling and administration of this complex infusion therapy.
The company also utilizes a direct sales force in key markets like the US and Europe to educate healthcare providers and build relationships, supported by scientific publications and major medical conferences to disseminate research findings and establish credibility.
Digital platforms, including the corporate website, serve as crucial hubs for investor relations and providing scientific data to healthcare professionals, with ongoing updates in 2024 reflecting transparency in pipeline progress and financial performance.
Strategic partnerships and licensing agreements are vital for expanding KIMMTRAK's global reach, leveraging partners' infrastructure for market entry and patient access in new territories.
| Channel | Description | Key Activities | 2023 Revenue Impact |
|---|---|---|---|
| Specialty Pharmacies & Treatment Centers | Curated distribution for KIMMTRAK | Handling, storage, administration | Contributed to $175.8M total revenue |
| Direct Sales Force (US, Europe) | Engaging healthcare providers | Physician education, relationship building | Essential for market penetration |
| Medical Conferences & Publications | Disseminating scientific research | Presenting clinical trial data (e.g., IMC-C103 at ASCO 2024) | Building scientific credibility, informing adoption |
| Digital Platforms (Website, Social Media) | Information hub for stakeholders | Investor relations, HCP resources, company news | Ensuring transparency and engagement in 2024 |
| Partnerships & Licensing | Expanding global reach | Market entry, leveraging partner infrastructure | Facilitating access in new territories |
Customer Segments
Adult patients diagnosed with unresectable or metastatic uveal melanoma (mUM) represent Immunocore's primary customer segment for KIMMTRAK. This rare and aggressive eye cancer, when it spreads and cannot be surgically removed, signifies a critical unmet medical need.
In 2024, the incidence of uveal melanoma in the United States was estimated to be around 6-8 cases per million people annually. For patients with metastatic uveal melanoma, treatment options have historically been very limited, underscoring the importance of therapies like KIMMTRAK.
Oncologists and melanoma specialists are critical customers, as they are the primary prescribers of KIMMTRAK. These healthcare professionals need robust clinical trial data, including efficacy and safety profiles, to confidently adopt new treatments. For instance, the Phase 3 KEYNOTE-716 study demonstrated that adjuvant pembrolizumab significantly improved recurrence-free survival in patients with resected stage III melanoma, providing strong evidence for similar immunotherapies.
Providing ongoing medical education and clear dosing guidelines is essential for these specialists to effectively integrate KIMMTRAK into their patient care pathways. They rely on peer-reviewed publications and expert consensus to inform their treatment decisions. In 2024, the focus remains on real-world data demonstrating KIMMTRAK's impact on patient outcomes and its place within the evolving melanoma treatment landscape.
Immunocore is actively expanding its reach to patients with other solid tumors, such as cutaneous melanoma, ovarian cancer, and non-small cell lung cancer (NSCLC). This represents a significant future customer segment where their ImmTAC technology could provide novel treatment avenues.
The company is making strides in this area, with ongoing clinical development targeting antigens like PRAME and PIWIL1, which are present in various solid tumor types. This pipeline expansion is crucial for broadening the applicability of their innovative therapeutic platform.
Specifically, Immunocore is currently enrolling patients in three Phase 3 trials focused on melanoma, including advanced cutaneous melanoma. This demonstrates a strong commitment to validating their ImmTAC technology in this specific solid tumor indication, with potential read-across to other solid tumor types.
Patients with Chronic Infectious Diseases (e.g., HIV, HBV)
Immunocore is expanding its focus to include patients battling chronic infectious diseases, such as HIV and Hepatitis B. This represents a significant opportunity to leverage their ImmTAV technology for conditions with substantial unmet medical needs.
The potential market for functional cures in these areas is vast. For instance, as of 2024, an estimated 39 million people globally were living with HIV, and over 250 million people were infected with Hepatitis B. Immunocore’s approach aims to offer a new therapeutic paradigm beyond current management strategies.
- Target Population: Patients with chronic infectious diseases like HIV and Hepatitis B.
- Therapeutic Goal: Achieve functional cures for these conditions.
- Market Potential: Addresses millions of individuals worldwide living with these chronic infections.
- Technology Application: Utilizes ImmTAV molecules to target and eliminate infected cells.
Patients with Autoimmune Diseases (e.g., Type 1 Diabetes, Atopic Dermatitis)
Immunocore is actively pursuing advancements in autoimmune diseases, including Type 1 Diabetes and Atopic Dermatitis, marking a significant expansion beyond its initial oncology focus. This strategic pivot leverages their proprietary ImmTAX technology, aiming to address the unmet needs of patients suffering from these chronic conditions.
The market for autoimmune disease treatments is substantial and growing. For instance, the global Type 1 Diabetes market was valued at approximately $7.1 billion in 2023 and is projected to reach $10.5 billion by 2030, growing at a CAGR of 5.7%. Similarly, the Atopic Dermatitis market is also experiencing robust growth, with an estimated size of $10.2 billion in 2023, expected to expand to $20.1 billion by 2030, at a CAGR of 10.1%.
- Target Patient Population: Individuals diagnosed with autoimmune conditions like Type 1 Diabetes and Atopic Dermatitis.
- Unmet Needs: Seeking more effective, long-term disease management and potential disease modification.
- Market Potential: Significant and expanding global markets with substantial revenue opportunities.
- Therapeutic Approach: Utilizing the ImmTAX platform to develop novel treatments for these debilitating diseases.
Immunocore's core customer segment comprises adult patients diagnosed with unresectable or metastatic uveal melanoma (mUM), a rare and aggressive cancer. Oncologists and melanoma specialists are key intermediaries, requiring robust clinical data to adopt KIMMTRAK.
The company is also targeting patients with other solid tumors, such as cutaneous melanoma, and those with chronic infectious diseases like HIV and Hepatitis B, aiming for functional cures. Furthermore, Immunocore is expanding into autoimmune diseases, including Type 1 Diabetes and Atopic Dermatitis, addressing significant unmet needs in these growing markets.
| Customer Segment | Target Condition | Key Need | Market Data (Illustrative 2024/2025) |
|---|---|---|---|
| mUM Patients | Metastatic Uveal Melanoma | Effective treatment for a rare, aggressive cancer | Incidence: 6-8 per million annually (US) |
| Oncologists/Specialists | Various Cancers | Efficacy, safety, and real-world data for treatment decisions | Focus on peer-reviewed publications and expert consensus |
| Solid Tumor Patients | Cutaneous Melanoma, NSCLC, etc. | Novel therapeutic avenues for difficult-to-treat cancers | Ongoing Phase 3 trials in advanced cutaneous melanoma |
| Infectious Disease Patients | HIV, Hepatitis B | Functional cures for chronic infections | HIV: ~39 million globally; Hep B: >250 million globally |
| Autoimmune Disease Patients | Type 1 Diabetes, Atopic Dermatitis | Improved disease management and modification | Type 1 Diabetes Market: ~$7.1B (2023) |
Cost Structure
Research and Development (R&D) represents a substantial cost for Immunocore, fueling its innovative ImmTAC platform. These expenditures encompass everything from early-stage preclinical research and drug discovery to the considerable financial commitment required for multiple phases of clinical trials for its promising pipeline candidates. In 2024, Immunocore reported R&D expenses of $222.2 million, underscoring the significant investment in future growth and platform expansion.
The company's continued investment in R&D is critical for driving innovation and broadening the application of its ImmTAC technology. For the first quarter of 2025, Immunocore's R&D expenses stood at $56.5 million, demonstrating an ongoing commitment to advancing its drug development programs.
Manufacturing and supply chain costs are significant for Immunocore, encompassing the intricate production of ImmTAC molecules, sourcing of raw materials, rigorous quality control, specialized packaging, and the complexities of global distribution. These expenses are a direct driver of the cost of goods sold for their flagship product, KIMMTRAK, and will similarly influence the economics of their future pipeline assets.
The production of advanced biologic therapies like ImmTACs is inherently capital-intensive. For instance, in 2023, Immunocore reported Cost of Sales of $124.8 million, a substantial portion of which is attributable to these manufacturing and supply chain elements. This highlights the substantial investment required to bring these innovative treatments from the lab to patients.
Selling, General, and Administrative (SG&A) expenses are a significant component of Immunocore's cost structure, covering essential commercialization activities. These costs include marketing initiatives to promote their therapies, salaries for a dedicated sales force, and the general administrative overhead necessary to run a global biopharmaceutical company. Legal and regulatory affairs are also critical elements within SG&A, ensuring compliance and market access.
As Immunocore continues to grow and introduce new treatments to the market, these SG&A expenses are projected to rise. This increase is directly tied to the investment required to build out commercial infrastructure, expand market reach, and support the successful launch and ongoing sales of their innovative therapies.
For the full year 2024, Immunocore reported SG&A expenses totaling $155.8 million. Looking at the first quarter of 2025, these costs amounted to $40.2 million, reflecting ongoing investment in commercial operations.
Clinical Trial Operations Costs
Clinical trial operations are a significant driver of Immunocore's cost structure. These expenses encompass critical activities such as patient recruitment, managing clinical sites, gathering and validating data, ongoing monitoring, and the eventual analysis of trial results. For instance, in 2023, Immunocore reported research and development expenses of $313.9 million, a substantial portion of which is allocated to these trial-related operations.
With multiple ongoing trials, including three in Phase 3 and several in earlier stages, these operational costs are not only substantial but also recurring. The complexity and duration of these trials directly influence the magnitude of these expenditures, making efficient management crucial for financial sustainability. The company's commitment to advancing its pipeline through rigorous clinical testing necessitates significant and continuous investment in these areas.
- Patient Recruitment: Costs associated with identifying, screening, and enrolling eligible participants.
- Site Management: Expenses for clinical sites, including investigator fees, site staff, and operational support.
- Data Collection & Monitoring: Costs for electronic data capture systems, data management, and clinical monitoring activities.
- Trial Analysis: Expenses related to statistical analysis, data interpretation, and reporting for regulatory submissions.
Intellectual Property and Legal Expenses
Intellectual property and legal expenses form a substantial cost center for Immunocore. These costs are directly tied to the protection and enforcement of their foundational ImmTAC technology and its applications. In 2023, the company reported significant spending in this area to safeguard its competitive advantage.
Maintaining and defending their extensive intellectual property portfolio, which includes numerous patent filings and ongoing renewals across key global markets, represents a considerable investment. This is crucial for securing market exclusivity for their innovative therapies.
- Patent Filings and Maintenance: Costs associated with preparing, filing, and maintaining patents globally to protect their ImmTAC technology and drug candidates.
- Intellectual Property Defense: Expenses incurred for defending their patents against potential infringements or challenges, which can include litigation.
- Regulatory Compliance: Legal costs related to navigating complex regulatory landscapes for drug development and approval in various jurisdictions.
- General Legal and Corporate Matters: Ongoing expenses for corporate legal advice, contracts, and other essential legal services.
Immunocore's cost structure is heavily weighted towards research and development, reflecting its commitment to innovation in the ImmTAC platform. Manufacturing and supply chain costs are also significant, driven by the complex production of its therapies like KIMMTRAK. The company also incurs substantial selling, general, and administrative expenses to support commercialization and global operations.
| Cost Category | 2024 (Millions) | Q1 2025 (Millions) |
| Research & Development | $222.2 | $56.5 |
| Cost of Sales (includes manufacturing) | $148.7 (2024 est.) | $37.2 (Q1 2025 est.) |
| Selling, General & Administrative | $155.8 | $40.2 |
Revenue Streams
Immunocore's primary revenue engine is the net sales of its groundbreaking drug, KIMMTRAK (tebentafusp). This therapy is specifically approved for patients battling unresectable or metastatic uveal melanoma, a rare and aggressive form of eye cancer. The company has seen significant uptake, with KIMMTRAK achieving $310.0 million in net sales for the entirety of 2024.
This sales performance highlights the successful market penetration of KIMMTRAK across key regions, including the United States and Europe, with ongoing launches in other international territories. The momentum continued into the first quarter of 2025, with KIMMTRAK generating an additional $93.9 million in net sales, demonstrating sustained demand and revenue growth for this critical oncology treatment.
Immunocore anticipates substantial future revenue from its diverse pipeline, which includes candidates targeting other solid tumors, infectious diseases, and autoimmune conditions. As these promising treatments progress through clinical trials and approach potential regulatory approval, they are poised to become significant revenue generators. This diversification strategy is crucial for reducing the company's dependence on any single product, thereby strengthening its long-term financial stability.
Collaboration and licensing agreements represent another avenue for revenue generation. These partnerships can involve upfront payments, milestone payments tied to specific development and regulatory successes, and ongoing royalties from eventual product sales.
In 2024, Immunocore reported collaboration revenue of $0.2 million. This marks a significant decrease from the $10.7 million recorded in 2023, indicating a shift in the timing or structure of these strategic partnerships.
Government Grants or Non-Dilutive Funding
Government grants and non-dilutive funding represent a crucial revenue stream for Immunocore, especially for programs addressing significant public health challenges. These funds reduce the reliance on equity financing, preserving shareholder value.
A prime example is the Bill & Melinda Gates Foundation's support for Immunocore's HIV program. This partnership highlights the company's ability to attract funding for critical research, demonstrating the potential for substantial non-dilutive capital infusion.
Such funding is vital for advancing early-stage research and development, allowing Immunocore to explore innovative therapeutic avenues without immediate pressure for commercial returns. It underpins the company's commitment to tackling diseases with broad societal impact.
- Government Grants: Funding from public bodies for specific R&D initiatives.
- Non-Dilutive Funding: Capital received without giving up equity, such as grants from foundations.
- HIV Program Support: Significant funding from the Bill & Melinda Gates Foundation for their HIV research.
- Strategic Importance: Reduces equity dilution and supports high-impact public health research.
Expanded Access/Compassionate Use Programs (Limited Revenue)
While not a core revenue stream, Immunocore can generate some income from expanded access or compassionate use programs. These programs offer patients with severe or life-threatening illnesses, who have no other treatment options, access to investigational therapies.
These initiatives are typically limited in scope and primarily serve to provide patient benefit rather than significant financial returns. For instance, in 2023, Immunocore's revenue was primarily driven by its commercial product KIMMTRAK, with expanded access programs contributing a smaller, supplementary amount.
- Limited Revenue Generation: Expanded access programs offer a pathway for generating some revenue, though it is not a primary focus.
- Patient-Centric Approach: These programs prioritize providing access to potentially life-saving treatments for patients with unmet medical needs.
- Regulatory Framework: Such programs operate under specific regulatory guidelines to ensure patient safety and ethical considerations.
- Supplementary Income: Revenue from these programs is supplementary to the main commercial activities and clinical development efforts.
Immunocore's revenue model is heavily reliant on the commercial success of its flagship drug, KIMMTRAK (tebentafusp). The company also leverages strategic partnerships and grants to fuel its research and development efforts.
While KIMMTRAK dominated 2024 with $310.0 million in net sales, collaboration revenue saw a significant dip to $0.2 million in 2024 from $10.7 million in 2023. This shift underscores the evolving nature of its revenue streams.
Future growth is anticipated from a robust pipeline of drug candidates and potential revenue from licensing agreements, alongside continued support from non-dilutive funding sources like government grants, exemplified by the Bill & Melinda Gates Foundation's support for its HIV program.
| Revenue Stream | 2024 Revenue (Millions USD) | 2023 Revenue (Millions USD) | Key Drivers |
|---|---|---|---|
| KIMMTRAK Net Sales | $310.0 | $175.7 | Uveal melanoma treatment uptake |
| Collaboration Revenue | $0.2 | $10.7 | Partnership agreements and milestones |
| Other (Grants, etc.) | Not specified | Not specified | Research funding, foundation support |
Business Model Canvas Data Sources
The Immunocore Business Model Canvas is informed by extensive clinical trial data, regulatory filings, and market analysis of the oncology and infectious disease sectors. These sources provide a robust foundation for understanding patient needs, competitive landscapes, and the economic viability of our therapeutic platforms.
