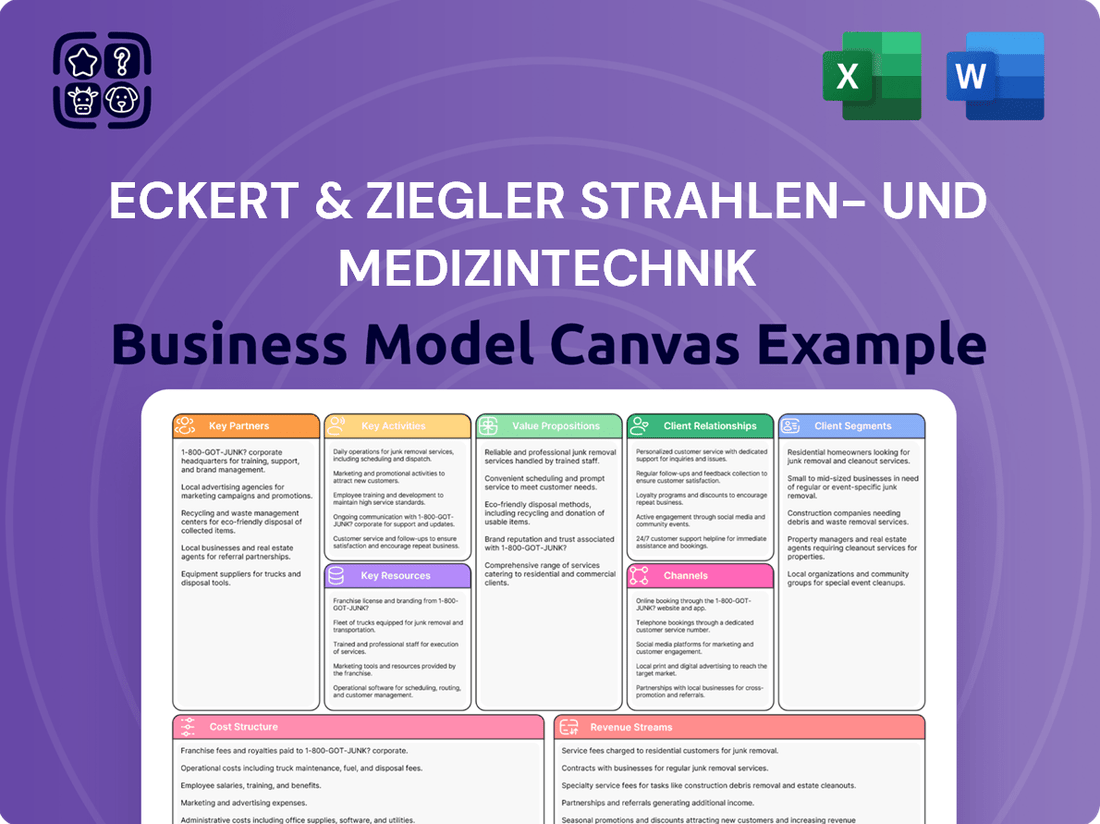
Eckert & Ziegler Strahlen- und Medizintechnik Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Eckert & Ziegler Strahlen- und Medizintechnik Bundle
Discover the strategic framework behind Eckert & Ziegler Strahlen- und Medizintechnik's success with our comprehensive Business Model Canvas. This detailed breakdown illuminates their approach to value creation, customer relationships, and revenue streams within the radiation and medical technology sectors. Unlock the full blueprint to understand their market positioning and competitive advantages.
Partnerships
Eckert & Ziegler's key partnerships with pharmaceutical companies and biotechs are foundational to its role in nuclear medicine. They supply vital radioisotopes such as Lutetium-177 (Lu-177) and Actinium-225 (Ac-225), which are essential for the development and manufacturing of innovative radiopharmaceuticals. These collaborations are critical for driving progress in targeted cancer therapies, a rapidly growing segment of nuclear medicine.
Recent strategic agreements highlight the significance of these relationships. For instance, partnerships with companies like AtomVie Global Radiopharma and GlyTherix ensure the steady supply of Lu-177 and Ac-225, respectively. Furthermore, an agreement with Bicycle Therapeutics underscores Eckert & Ziegler's integrated approach, covering both radioisotope provision and contract development and manufacturing for their Bicycle Radio Conjugates.
Eckert & Ziegler's collaborations with research institutions and universities are crucial for maintaining its leadership in isotope technology. These partnerships facilitate joint R&D, clinical trials, and knowledge sharing, ensuring their radiopharmaceutical products align with scientific advancements and regulatory standards. For instance, in 2024, the company continued its engagement with several academic centers, supporting early-stage research into novel diagnostic and therapeutic agents.
Eckert & Ziegler operates as a Contract Manufacturing Organization (CMO), offering specialized production and distribution of radiopharmaceuticals to other businesses. This service is crucial for companies lacking their own advanced manufacturing infrastructure.
A prime example is their collaboration with ARTBIO, where Eckert & Ziegler facilitates the manufacturing and delivery of novel therapies. This partnership specifically leverages Eckert & Ziegler's expertise in isolating isotopes like Lead-212 (Pb-212), a key component in targeted alpha therapies.
By utilizing Eckert & Ziegler's extensive global CMO network, partners like ARTBIO can accelerate the development and commercialization of their radiopharmaceutical products. This global reach ensures wider accessibility and efficient supply chains for these critical medical treatments.
Medical Device Manufacturers
Eckert & Ziegler's collaborations with medical device manufacturers are crucial for embedding their radioactive components into sophisticated delivery systems. This integration is vital for applications in cancer therapy, such as brachytherapy, and for nuclear medicine. For instance, partnerships ensure that products like their brachytherapy seeds and ophthalmic applicators function optimally within the larger therapeutic solutions offered by these manufacturers.
These alliances are not just about component compatibility; they also drive innovation. By working closely with device makers, Eckert & Ziegler can tailor their radioactive materials and isotopes to meet the specific design and performance requirements of cutting-edge medical equipment. This symbiotic relationship allows for the development of more effective and targeted treatments.
- Integration of Radioactive Components: Eckert & Ziegler's radioactive isotopes and sources are integrated into specialized delivery systems for cancer therapy and nuclear medicine, ensuring seamless functionality within the broader treatment solutions provided by their partners.
- Ensuring Compatibility and Performance: Partnerships with medical device manufacturers guarantee that products like brachytherapy seeds and ophthalmic applicators are compatible with and perform optimally within the delivery devices, contributing to improved patient outcomes.
- Collaboration with Brachytherapy Device Companies: Specific collaborations exist with companies specializing in brachytherapy devices, facilitating the development and refinement of localized radiation treatment technologies.
- Market Presence and Reach: These key partnerships expand Eckert & Ziegler's market reach by making their specialized components accessible through established medical device platforms, thereby increasing their impact on patient care globally.
Regulatory Bodies and Certification Agencies
Eckert & Ziegler's engagement with regulatory bodies and certification agencies is foundational to its operations. Securing certifications, like the MDR certification for its prostate seeds, is paramount for market entry and sustained product availability. This adherence to strict safety and quality standards, as exemplified by their commitment to regulatory excellence, directly impacts patient safety and market trust.
These partnerships are not merely about compliance; they are strategic enablers. For instance, the Medical Device Regulation (MDR) in Europe, which came into full effect in May 2021, imposes rigorous requirements for product safety and performance. Eckert & Ziegler's successful navigation of these regulations, including obtaining MDR certification for key products, demonstrates a robust quality management system and a commitment to meeting evolving global healthcare standards. This proactive approach is vital for maintaining access to critical markets and ensuring the long-term viability of their medical technology offerings.
- Regulatory Adherence: Eckert & Ziegler actively partners with bodies like the FDA in the US and European notified bodies to ensure its products meet stringent safety and efficacy standards.
- Certification Milestones: Obtaining certifications such as the MDR for its prostate brachytherapy seeds is a critical step, enabling market access in the European Union and reinforcing product quality.
- Market Access and Trust: Compliance with these regulations fosters trust among healthcare providers and patients, ensuring the continued availability of their radiopharmaceutical and medical device products globally.
- Quality Assurance: These partnerships underscore Eckert & Ziegler's dedication to maintaining high-quality manufacturing processes and product integrity, crucial for patient care.
Eckert & Ziegler's key partnerships with pharmaceutical and biotech firms are central to its nuclear medicine business, supplying essential radioisotopes like Lutetium-177 and Actinium-225 for targeted cancer therapies. These collaborations, including recent agreements with AtomVie Global Radiopharma and Bicycle Therapeutics in 2024, ensure a reliable supply chain and support the development of innovative radiopharmaceuticals.
What is included in the product
This Business Model Canvas provides a detailed overview of Eckert & Ziegler Strahlen- und Medizintechnik's operations, focusing on their specialized medical technology and radiation applications.
It outlines key customer segments, value propositions, and revenue streams, reflecting the company's strategic positioning in niche healthcare markets.
Eckert & Ziegler's Business Model Canvas offers a clear, structured way to visualize their operations, helping to alleviate the pain point of complex strategy by presenting it in a digestible, one-page snapshot.
Activities
Eckert & Ziegler is deeply invested in advancing isotope technologies, particularly for medical breakthroughs. Their research focuses on developing novel radioactive components for a range of applications.
A significant area of focus is the pioneering development of Good Manufacturing Practice (GMP)-grade Actinium-225 (Ac-225), a critical isotope for targeted alpha therapy in cancer treatment. They are also enhancing the applications of Lutetium-177 (Lu-177), another vital isotope for radiopharmaceutical therapies.
This commitment to continuous innovation, exemplified by their work on Ac-225 and Lu-177, is crucial for maintaining a competitive edge and addressing critical unmet needs in advanced medical treatments. For instance, the demand for Lu-177-based therapies has seen substantial growth, with market projections indicating continued expansion in the coming years, underscoring the importance of Eckert & Ziegler's R&D efforts in this space.
Eckert & Ziegler's core activity is the meticulous production and manufacturing of diverse radioactive components. This includes vital products like brachytherapy seeds for cancer treatment, various radioisotopes essential for diagnostics and therapy, and radiopharmaceuticals used in nuclear medicine. The company manages intricate global supply chains for specialized raw materials, ensuring a consistent flow for its advanced manufacturing processes.
Operating highly specialized facilities, Eckert & Ziegler undertakes large-scale production to address worldwide demand for its radioactive products. This commitment to meeting global needs is underscored by ongoing capacity expansion. For instance, investments in new cyclotron facilities are a key part of their strategy to bolster production capabilities and support anticipated future growth in the radiopharmaceutical and isotope markets.
Eckert & Ziegler excels in managing a sophisticated global distribution network and supply chain, ensuring the secure and prompt delivery of its radioactive products to a worldwide customer base. This critical function demands expertise in handling hazardous materials and maintaining streamlined logistics to support both early and late-stage development projects for its clients.
The company's strategic positioning with manufacturing facilities in key locations like Berlin, Boston, and Jintan, China, significantly enhances its ability to efficiently distribute its specialized products across diverse international markets. This global infrastructure is vital for meeting the demanding timelines inherent in the pharmaceutical and medical technology sectors.
Provision of Radiation Protection and Analysis Services
Eckert & Ziegler extends its expertise beyond manufacturing by offering crucial radiation protection and analysis services. These services are vital for clients to safely manage, transport, and utilize radioactive materials, ensuring regulatory compliance and operational integrity.
This service offering complements their isotope technology core, providing a comprehensive solution for customers. For instance, in 2023, the company reported significant revenue growth in its Isotope Products segment, which heavily relies on these supporting services to maintain its market position.
- Radiation Safety Consulting
- Dosimetry and Calibration Services
- Environmental Monitoring and Analysis
- Custom Shielding Solutions
Clinical Development and Regulatory Affairs
Eckert & Ziegler's clinical development and regulatory affairs are crucial for market entry and ongoing product viability. The company actively supports clinical trials for novel radiopharmaceuticals, a key area for future growth.
Securing necessary approvals is paramount. For instance, Eckert & Ziegler achieved EMA approval for Theralugand® (Lu-177), a significant milestone for their radiopharmaceutical offerings. Furthermore, they obtained MDR certification for their brachytherapy products, demonstrating compliance with stringent European medical device regulations.
- Clinical Trials: Supporting the advancement of new radiopharmaceutical treatments through rigorous testing.
- Regulatory Approvals: Obtaining essential clearances like EMA approval for Theralugand® (Lu-177).
- Product Certification: Ensuring compliance with standards such as MDR certification for brachytherapy devices.
- Market Access: Facilitating the global launch and continued availability of innovative medical technologies.
Eckert & Ziegler's key activities revolve around the meticulous production and global distribution of specialized radioactive components and radiopharmaceuticals. They are deeply involved in research and development, particularly focusing on advanced isotopes like Actinium-225 for targeted alpha therapy and Lutetium-177 for radiopharmaceutical treatments. This includes managing complex supply chains and operating specialized manufacturing facilities to meet worldwide demand, exemplified by their ongoing investments in cyclotron capacity to support market growth.
| Key Activity | Description | Impact/Data Point |
|---|---|---|
| Isotope Production & Manufacturing | Producing radioisotopes for medical diagnostics and therapy, including brachytherapy seeds and radiopharmaceuticals. | The Isotope Products segment is a significant revenue driver, with reported substantial growth in 2023, reflecting strong demand. |
| Research & Development | Developing novel radioactive components and advancing isotope technologies, such as GMP-grade Ac-225 and Lu-177 applications. | Commitment to R&D is crucial for maintaining a competitive edge and addressing unmet needs in advanced medical treatments. |
| Global Distribution & Supply Chain Management | Ensuring secure and timely delivery of radioactive products worldwide, managing hazardous materials logistics. | Strategic manufacturing locations in Berlin, Boston, and Jintan enhance efficient distribution across international markets. |
| Radiation Protection & Analysis Services | Providing services for safe management, transport, and utilization of radioactive materials, ensuring regulatory compliance. | These services complement core offerings and support the market position of their isotope technologies. |
| Clinical Development & Regulatory Affairs | Supporting clinical trials for radiopharmaceuticals and securing regulatory approvals for market entry. | Achieved EMA approval for Theralugand® (Lu-177) and MDR certification for brachytherapy products, demonstrating regulatory success. |
Full Version Awaits
Business Model Canvas
The Eckert & Ziegler Strahlen- und Medizintechnik Business Model Canvas preview you are viewing is the actual document you will receive upon purchase. This means you are seeing the complete, professionally structured content and layout that will be yours to use immediately. There are no mockups or samples; what you see is precisely the file you will download, ready for your strategic planning and analysis.
Resources
Eckert & Ziegler's proprietary isotope production and processing technologies are a cornerstone of their business model, enabling the creation of highly sought-after radioisotopes for medical use. Their advanced methods allow for the generation of non-carrier-added Actinium-225 (Ac-225) and Lutetium-177 (Lu-177), crucial components in targeted radiotherapies. This technological edge directly translates into a competitive advantage, ensuring the supply of high-quality isotopes that meet stringent pharmaceutical standards.
A key differentiator is their AlphaDirect™ Lead-212 isolation technology, which significantly enhances the efficiency and purity of Lead-212 production. This specialized capability is vital for the development of next-generation alpha-targeted cancer treatments. By controlling these sophisticated production processes, Eckert & Ziegler secures its position as a leading supplier in the growing radiopharmaceutical market.
Eckert & Ziegler Strahlen- und Medizintechnik's highly specialized manufacturing facilities, including Good Manufacturing Practice (GMP)-compliant sites and cyclotrons, are critical. These facilities are outfitted with advanced infrastructure like hot cells, enabling the safe and efficient production of radioactive components and radiopharmaceuticals that adhere to strict regulatory standards.
The company's ongoing investment in capacity expansion, exemplified by its new Lutetium production facility, underscores the strategic importance of these specialized manufacturing assets. For instance, the company announced in 2023 plans to expand its cyclotron capacity in Berlin, highlighting a commitment to enhancing its production capabilities for radiopharmaceutical precursors.
Eckert & Ziegler's success hinges on its highly skilled scientific and technical personnel. This team, comprising over 1,000 employees as of 2024, possesses deep expertise in critical areas like nuclear medicine, radiochemistry, and radiation therapy.
This intellectual capital is the engine behind the company's innovation and ensures the high quality of its products and services. Their specialized knowledge is essential for developing advanced solutions in radiation protection and analysis, directly supporting Eckert & Ziegler's core business.
Licenses, Certifications, and Regulatory Approvals
Eckert & Ziegler's extensive portfolio of licenses, certifications, and regulatory approvals, such as MDR certification and EMA approval for Theralugand®, are critical resources. These accreditations are essential for legal operation and global product sales, signifying adherence to stringent international quality and safety benchmarks. This compliance fosters market trust and ensures continued access to key markets.
Maintaining these approvals is an ongoing process, requiring continuous updates and vigilance. For instance, in 2024, the company would have been actively managing its compliance with evolving medical device regulations across various jurisdictions. These certifications are not static; they represent a dynamic commitment to upholding the highest industry standards, which is fundamental to their business model.
- MDR Certification: Essential for market access within the European Economic Area.
- EMA Approval: Specifically for products like Theralugand®, crucial for pharmaceutical applications in Europe.
- Global Regulatory Compliance: Navigating and maintaining approvals from bodies like the FDA (USA) and other national health authorities.
- Quality Management Systems: Certifications like ISO 13485 underscore their commitment to quality throughout the product lifecycle.
Intellectual Property (Patents and Know-how)
Eckert & Ziegler's intellectual property, particularly its patents covering innovative isotope production methods, represents a core intangible asset. This IP is crucial for maintaining their competitive edge in the niche market of isotope technology.
The company's proprietary know-how in radiopharmaceutical development, especially concerning Ga-68 generators and Lu-177, is a key differentiator. This expertise allows them to offer specialized solutions in targeted cancer therapies.
- Patented Isotope Production: Eckert & Ziegler holds patents on advanced methods for producing isotopes essential for medical imaging and therapy.
- Radiopharmaceutical Expertise: The company possesses significant proprietary know-how in the development and manufacturing of radiopharmaceuticals, including Ga-68 generators and Lu-177-based therapies.
- Competitive Advantage: This intellectual property portfolio safeguards their innovations and provides a distinct advantage in the highly specialized field of nuclear medicine and isotope technology.
Eckert & Ziegler's key resources include their advanced, proprietary isotope production and processing technologies, such as AlphaDirect™ for Lead-212, enabling the creation of critical radioisotopes like Actinium-225 and Lutetium-177. Their highly specialized manufacturing facilities, including GMP-compliant sites and cyclotrons, are essential for safe and efficient production of radioactive components. Furthermore, the company's intellectual property, encompassing patents on isotope production methods and deep radiopharmaceutical know-how, forms a crucial intangible asset, safeguarding their innovations and competitive edge in the nuclear medicine sector.
| Resource Category | Specific Resource | Significance | 2024 Data/Context |
|---|---|---|---|
| Technology & Processes | Isotope Production & Processing | Enables creation of sought-after radioisotopes (Ac-225, Lu-177) for targeted therapies. | Proprietary methods for non-carrier-added isotopes. |
| Technology & Processes | AlphaDirect™ Lead-212 Isolation | Enhances efficiency and purity of Lead-212 for alpha-targeted treatments. | Key differentiator for next-generation cancer therapies. |
| Physical Assets | GMP-Compliant Manufacturing Facilities | Crucial for safe, compliant production of radioactive components and radiopharmaceuticals. | Includes hot cells and advanced infrastructure. |
| Physical Assets | Cyclotrons | Essential for producing radiopharmaceutical precursors. | Ongoing capacity expansion, e.g., Berlin facility. |
| Human Capital | Skilled Scientific & Technical Personnel | Deep expertise in nuclear medicine, radiochemistry, radiation therapy. | Over 1,000 employees in 2024, driving innovation. |
| Intellectual Property | Patents on Isotope Production Methods | Maintains competitive edge in specialized isotope technology market. | Protects proprietary innovations. |
| Intellectual Property | Radiopharmaceutical Know-How | Expertise in Ga-68 generators and Lu-177 therapies. | Provides specialized solutions in targeted cancer therapies. |
| Regulatory & Legal | Licenses, Certifications, Approvals | Enables legal operation and global product sales (e.g., MDR, EMA). | Ensures adherence to international quality and safety standards; ongoing compliance management. |
Value Propositions
Eckert & Ziegler provides premium radioactive components, specifically designed for vital medical, scientific, and industrial uses. Their dedication to accuracy and dependability is evident in products like non-carrier added Lutetium-177 and Actinium-225, which are crucial for cutting-edge treatments and research.
This focus on quality is backed by their adherence to strict regulatory standards, ensuring their components meet the highest industry benchmarks.
Eckert & Ziegler offers sophisticated solutions for cancer treatment, notably with its brachytherapy products, and for nuclear medicine, supplying both diagnostic and therapeutic radioisotopes. These advanced offerings are crucial for delivering precise and effective treatments, thereby reducing harm to healthy surrounding tissues.
The company's portfolio directly addresses the growing need for personalized medicine within oncology. In 2024, the global cancer diagnostics market, which includes nuclear medicine imaging, was projected to reach approximately $25 billion, highlighting the significant demand for the types of solutions Eckert & Ziegler provides.
Eckert & Ziegler provides a complete suite of services covering the entire radiopharmaceutical lifecycle. This integrated model supports partners from initial research and development through contract manufacturing, distribution, and essential radiation protection measures. For instance, in 2024, the company continued to invest in its manufacturing capabilities, aiming to meet the growing demand for radiopharmaceuticals.
This end-to-end offering simplifies the complex journey of developing and commercializing radiopharmaceutical therapies. By consolidating these critical functions, Eckert & Ziegler enhances convenience and reliability for its clients, accelerating the path to market for innovative treatments. Their portfolio includes essential infrastructure, such as specialized hot cells, crucial for safe and efficient radiopharmaceutical handling.
Reliable Supply and Global Reach
Eckert & Ziegler's commitment to reliable supply is underscored by its extensive global manufacturing presence, with key sites strategically located across Europe, North America, and Asia. This distributed network, coupled with robust supply agreements, significantly reduces the vulnerability to disruptions, a critical factor in the consistent delivery of radioisotopes essential for medical diagnostics and treatments. In 2024, the company continued to invest in expanding its production capacities, aiming to meet the growing global demand for radiopharmaceuticals.
Their global reach ensures that vital medical isotopes are accessible to healthcare providers and patients worldwide. This expansive network is not just about distribution; it's about building resilience. By diversifying manufacturing and sourcing, Eckert & Ziegler safeguards against localized issues, maintaining an uninterrupted flow of products. This focus on supply chain integrity is a cornerstone of their value proposition in the highly regulated and time-sensitive nuclear medicine sector.
- Global Manufacturing Footprint: Operations in multiple continents ensure broad accessibility.
- Supply Chain Resilience: Diversified production and partnerships mitigate disruption risks.
- Strategic Expansion: Ongoing investments in 2024 bolster future supply capabilities.
- Critical Product Delivery: Ensuring consistent access to radioisotopes for global healthcare.
Expertise in Radiation Protection and Safety
Eckert & Ziegler's deep expertise in radiation protection and safety is a cornerstone of its value proposition. This specialized knowledge ensures clients can handle, transport, and utilize radioactive materials with the utmost security and compliance. This is critical in an industry where safety is paramount and regulatory adherence is non-negotiable.
This commitment to safety acts as a significant differentiator for Eckert & Ziegler. Their rigorous adherence to safety protocols and regulatory frameworks provides peace of mind and operational integrity for their customers. This expertise extends to comprehensive analysis services, further solidifying their role as a trusted partner.
- Unwavering Commitment to Safety: Eckert & Ziegler's core strength lies in its profound understanding and application of radiation protection and safety standards.
- Regulatory Assurance: Clients benefit from guaranteed compliance with stringent industry regulations, mitigating risks associated with radioactive materials.
- Comprehensive Analysis Services: Beyond handling and transport, the company offers detailed analytical services related to radiation safety.
- Industry Leadership: This focus on safety and expertise positions Eckert & Ziegler as a leader in a highly specialized and regulated sector.
Eckert & Ziegler excels in providing high-purity radioactive components, essential for advancements in nuclear medicine and industrial applications, with a particular focus on isotopes like Lutetium-177. Their value lies in delivering precisely engineered, reliable materials that are critical for both innovative cancer therapies and advanced scientific research, ensuring consistent quality for demanding applications.
The company offers a comprehensive, end-to-end service model that guides clients through the entire radiopharmaceutical lifecycle, from R&D to manufacturing and radiation protection. This integrated approach streamlines the complex process of bringing radiopharmaceutical treatments to market, offering a single point of contact for critical development and production needs.
With a robust global manufacturing presence and a commitment to supply chain resilience, Eckert & Ziegler ensures the consistent availability of vital radioisotopes worldwide. Their strategic investments in expanding production capacity, as seen in 2024, underscore their dedication to meeting the escalating global demand for these critical medical supplies.
Eckert & Ziegler's deep expertise in radiation protection and safety provides clients with unparalleled assurance in handling, transporting, and utilizing radioactive materials. This focus on stringent safety protocols and regulatory compliance positions them as a trusted leader, offering peace of mind and operational integrity in a highly regulated industry.
| Value Proposition | Key Offering | Target Need | 2024 Relevance |
|---|---|---|---|
| Premium Radioactive Components | High-purity isotopes (e.g., Lu-177) | Cutting-edge medical treatments & research | Growing demand in oncology |
| End-to-End Radiopharmaceutical Services | R&D to distribution support | Streamlined therapy development | Investment in manufacturing capacity |
| Global Supply Chain Resilience | Distributed manufacturing, risk mitigation | Consistent access to vital isotopes | Meeting escalating global demand |
| Radiation Protection & Safety Expertise | Compliance, secure handling solutions | Regulatory assurance & operational integrity | Industry leadership in specialized sector |
Customer Relationships
Eckert & Ziegler cultivates direct sales channels, fostering personalized interactions with clients to understand their unique requirements. This approach ensures that customers receive tailored solutions and expert guidance throughout their engagement.
Comprehensive technical support is a cornerstone of their customer relationships, offering immediate assistance for product usage, troubleshooting, and optimization. This direct support model, exemplified by their commitment to responsive service, aims for high customer satisfaction and seamless integration of their advanced technologies.
Eckert & Ziegler cultivates enduring partnerships with pharmaceutical firms, research bodies, and other key industry stakeholders. These collaborations frequently extend to joint development initiatives, the co-creation of specialized solutions, and strategic supply arrangements, underscoring a dedication to shared advancement and innovation within the radiopharmaceutical sector.
These deep-rooted relationships are fundamental to Eckert & Ziegler's operational model, fostering trust and enabling the development of cutting-edge radiopharmaceutical technologies. For instance, in 2024, the company continued to solidify its position through strategic alliances that facilitated the expansion of its product pipeline and market reach, directly impacting its revenue growth in specialized medical isotopes.
Eckert & Ziegler employs a specialized consultative approach, providing expert guidance and tailored solutions for clients in medical, scientific, and industrial fields. This involves deep dives into isotope applications, regulatory adherence, and custom product development, helping clients navigate the complex world of isotope technology.
Dedicated Investor Relations and Transparency
Eckert & Ziegler Strahlen- und Medizintechnik prioritizes clear communication with its investors through a dedicated investor relations team. This function ensures that a wide range of stakeholders, from individual investors to financial professionals, receive timely and accurate information. For instance, their 2023 annual report provided detailed insights into their revenue streams and strategic outlook.
Transparency is a cornerstone of their approach, with regular updates that include detailed annual and quarterly financial reports. They also conduct earnings calls and provide financial forecasts. This commitment helps build trust and empowers stakeholders to make well-informed investment decisions. In 2023, the company reported revenue of €203.7 million, demonstrating growth and stability.
- Dedicated Investor Relations: A specialized team manages communication with all investor types.
- Transparent Reporting: Regular publication of annual and quarterly reports, earnings calls, and financial forecasts.
- Access to Critical Data: Ensuring stakeholders can easily access essential financial information for decision-making.
- Fostering Trust: Building confidence through consistent and open communication practices.
Customer Training and Education
Eckert & Ziegler provides comprehensive training and educational programs. These initiatives ensure customers can safely and effectively operate their specialized medical and scientific equipment. For instance, in 2024, the company continued to emphasize hands-on workshops for its isotope production and radiation therapy devices, aiming to minimize user error and maximize treatment efficacy.
This focus on customer capability and confidence is crucial, especially given the complexity of their product applications. By reinforcing best practices, Eckert & Ziegler enhances the perceived value of their solutions, leading to greater customer loyalty and satisfaction.
- Enhanced Product Proficiency: Customers gain the skills to utilize Eckert & Ziegler's advanced technology correctly.
- Safety and Compliance: Training ensures adherence to stringent safety protocols in handling radioactive materials and medical devices.
- Improved Patient Outcomes: For medical applications, educated users contribute to more effective and safer patient treatments.
- Long-Term Partnership: Investing in customer education fosters a stronger, collaborative relationship beyond the initial sale.
Eckert & Ziegler fosters deep, collaborative relationships with key industry partners, including pharmaceutical companies and research institutions. These collaborations often involve joint development projects and tailored supply agreements, driving innovation in radiopharmaceuticals. In 2024, the company continued to strengthen these alliances, which are vital for expanding its product portfolio and market reach, directly contributing to revenue growth in specialized medical isotopes.
| Customer Segment | Relationship Type | Key Activities | 2024 Focus |
|---|---|---|---|
| Pharmaceutical Firms & Research Bodies | Co-creation & Strategic Partnerships | Joint development, tailored supply, shared advancement | Expanding product pipeline through alliances |
| Medical Professionals & Scientists | Consultative & Training | Expert guidance, application support, safety training | Enhancing user proficiency and safety protocols |
| Investors | Transparent Communication | Financial reporting, earnings calls, investor relations | Maintaining trust through consistent data access |
Channels
Eckert & Ziegler leverages a dedicated direct sales force to engage specialized clients like hospitals, research facilities, and pharmaceutical firms. This direct approach is crucial for building strong relationships and offering customized solutions, especially for significant contracts and intricate product lines. In 2024, this strategy allowed them to maintain close ties with key partners in the medical technology sector.
Eckert & Ziegler's global distribution network is a cornerstone of its business, ensuring its specialized products reach customers across continents. This network is vital for the timely and secure delivery of radioactive components and radiopharmaceuticals, supporting critical applications in medicine and research.
With manufacturing and distribution hubs strategically located in America, Europe, and Asia, the company effectively serves a diverse international clientele. This expansive reach allows Eckert & Ziegler to maintain a consistent supply chain, crucial for ongoing clinical trials and commercial radiopharmaceutical operations.
In 2024, the company's commitment to this robust network facilitated the distribution of its products to over 100 countries, underscoring its significant global footprint in the nuclear medicine sector.
Eckert & Ziegler leverages strategic partnerships and collaborations as a key channel to broaden its market presence and deliver its specialized radiopharmaceutical products and services. For instance, collaborations with entities like AtomVie Global Radiopharma and ARTBIO are instrumental in this expansion.
These alliances are designed to unlock access to new geographical markets and customer bases, particularly for the intricate development and manufacturing of radiopharmaceuticals. Such strategic moves directly contribute to enhancing the company's overall global footprint and market penetration.
Industry Conferences and Scientific Publications
Eckert & Ziegler actively participates in key industry conferences, such as the SNMMI Annual Meeting and the ESTRO Congress. These events are crucial for demonstrating their latest advancements in radiopharmaceuticals and medical devices to a targeted audience of nuclear medicine physicians, radiation oncologists, and industry professionals.
Publishing in peer-reviewed scientific journals, including the Journal of Nuclear Medicine and Radiotherapy and Oncology, further solidifies Eckert & Ziegler's position as a thought leader. In 2024, the company continued to contribute research findings that validate the efficacy and safety of their products, reaching a global network of researchers and clinicians.
- Industry Conferences: Eckert & Ziegler regularly exhibits at major global events like the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting and the European Society for Radiotherapy and Oncology (ESTRO) Congress.
- Scientific Publications: The company's research is featured in leading journals such as the Journal of Nuclear Medicine and Radiotherapy and Oncology, showcasing clinical data and technological innovations.
- Brand Visibility and Thought Leadership: These channels are vital for enhancing brand recognition and establishing Eckert & Ziegler as a trusted authority in nuclear medicine and radiation therapy.
- Customer and Partner Engagement: Participation facilitates direct interaction with key decision-makers, fostering new business opportunities and strengthening existing relationships.
Online Presence and Investor Relations Portal
Eckert & Ziegler Strahlen- und Medizintechnik leverages its corporate website and a dedicated investor relations portal as key channels for transparent communication. This digital presence disseminates financial results, product updates, and corporate news, reaching a broad spectrum of investors and analysts.
These platforms are crucial for fostering informed decision-making among stakeholders. For instance, in 2024, the company reported a significant increase in website traffic to its investor section following the release of its Q3 earnings, highlighting the portal's effectiveness in engaging the financial community.
- Corporate Website: Serves as a primary hub for company information, including annual reports and press releases.
- Investor Relations Portal: Offers dedicated sections for financial data, stock information, and shareholder communications.
- Information Dissemination: Facilitates timely updates on financial performance and strategic developments.
- Stakeholder Engagement: Supports informed decision-making by providing accessible and comprehensive data.
Eckert & Ziegler's distribution strategy is multi-faceted, encompassing direct sales, a global network, strategic partnerships, and digital platforms. In 2024, their global distribution network facilitated product delivery to over 100 countries, demonstrating a significant international reach.
Strategic alliances with companies like AtomVie Global Radiopharma and ARTBIO are crucial for expanding market access, particularly in the radiopharmaceutical sector. The company also actively engages with its audience through participation in industry conferences and scientific publications, reinforcing its market presence and thought leadership.
Their digital channels, including the corporate website and investor relations portal, are vital for transparent communication with investors and analysts. In 2024, the investor relations portal saw increased traffic following earnings releases, indicating its effectiveness in engaging the financial community.
| Channel | Description | 2024 Impact/Focus |
|---|---|---|
| Direct Sales Force | Engages specialized clients (hospitals, research, pharma) for customized solutions. | Maintained strong ties with key medical technology partners. |
| Global Distribution Network | Ensures timely and secure delivery of radioactive components worldwide. | Served over 100 countries, vital for clinical trials and operations. |
| Strategic Partnerships | Collaborations to access new markets and customer bases for radiopharmaceuticals. | Unlocking new markets through alliances like AtomVie and ARTBIO. |
| Industry Conferences & Publications | Showcases advancements and validates product efficacy to professionals. | Published research in journals and presented at SNMMI and ESTRO meetings. |
| Digital Platforms (Website, Investor Portal) | Disseminates financial results, product updates, and corporate news. | Increased investor portal traffic post-earnings releases. |
Customer Segments
Hospitals and specialized cancer treatment centers are primary customers for Eckert & Ziegler's medical technologies. These institutions deploy the company's brachytherapy products for precise, localized cancer treatments, targeting conditions like prostate, brain, and eye tumors. They also utilize Eckert & Ziegler's radiopharmaceuticals, which are crucial for both diagnostic imaging, allowing for early disease detection, and therapeutic applications, directly treating cancerous cells.
The demand from this segment is significantly influenced by the global rise in cancer incidence. For instance, the World Health Organization projected over 28 million cancer cases annually by 2040, a substantial increase from recent years. This growing patient population directly translates into higher utilization of brachytherapy and radiopharmaceutical treatments, underscoring the critical role these healthcare providers play in Eckert & Ziegler's market.
Pharmaceutical and biotechnology firms are key clients, especially those focused on radiopharmaceutical development. Eckert & Ziegler provides them with essential radioisotopes and contract manufacturing, vital for bringing new therapies from research to market.
In 2024, the global radiopharmaceutical market was valued at approximately $7.5 billion, with significant growth driven by advancements in targeted therapies and diagnostics. Eckert & Ziegler’s role in supplying radioisotopes and offering manufacturing services directly supports this expanding sector, enabling companies to conduct clinical trials and scale production of life-saving treatments.
Research institutions and academic laboratories are key consumers of Eckert & Ziegler's offerings, utilizing their radioactive components and services for critical scientific endeavors. This includes vital work in drug discovery, where precise isotopes are essential for tracing and testing. In 2024, the demand for specialized radioisotopes for preclinical research remained robust, underscoring the segment's reliance on high-quality, reliable materials for experimental applications.
Industrial Clients
Industrial clients rely on Eckert & Ziegler's isotope products for critical applications like industrial gauging, precise measurement, and environmental analysis. These customers need dependable radiation sources to ensure quality control, streamline processes, and maintain safety standards across diverse sectors. For example, in 2024, the industrial gauging market, a key area for isotope use, continued to see steady demand driven by manufacturing efficiency needs.
This segment values the reliability and accuracy of Eckert & Ziegler's offerings for tasks such as non-destructive testing and process monitoring. The company's ability to provide specialized isotope solutions underscores the wide-ranging utility of their core technology. The global market for radioisotopes, which includes industrial applications, was projected to grow, reflecting sustained industrial investment in advanced measurement techniques.
- Industrial Gauging: Essential for quality control and process optimization in manufacturing.
- Measurement and Analysis: Used in scientific research and industrial diagnostics.
- Environmental Monitoring: Crucial for tracking pollution and ensuring safety.
- Reliability and Precision: Key requirements for industrial clients dependent on accurate data.
Government Agencies and Regulatory Bodies
Government agencies and regulatory bodies are vital stakeholders, shaping the market for radioactive materials and medical technology through regulations, licensing, and the establishment of industry standards. Eckert & Ziegler actively engages with these entities to ensure full compliance with all legal frameworks, contributing to the development of robust industry standards, and addressing critical public health and safety concerns. This proactive engagement is fundamental to maintaining operational integrity and trust within the sector.
For instance, in 2024, regulatory approvals for new medical devices incorporating radioactive isotopes often involve extensive consultation with national health authorities and international atomic energy agencies. These bodies set stringent safety protocols and quality management systems that companies like Eckert & Ziegler must adhere to, impacting product development timelines and market access. The company's commitment to meeting these evolving regulatory landscapes, such as those overseen by the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA), is a cornerstone of its business model.
- Regulatory Compliance: Ensuring adherence to national and international regulations governing the use and handling of radioactive materials, critical for market access and operational legality.
- Standard Setting: Participating in the development and refinement of industry standards for safety, quality, and efficacy in radiopharmaceuticals and medical technology.
- Public Health and Safety: Addressing and mitigating risks associated with radioactive materials to protect public health and the environment, a key responsibility for all stakeholders.
- Licensing and Approvals: Navigating and securing necessary licenses and product approvals from governmental bodies, a prerequisite for commercialization and distribution.
Eckert & Ziegler's customer base is diverse, encompassing healthcare providers, research entities, and industrial sectors. Hospitals and specialized cancer treatment centers are primary users of their brachytherapy and radiopharmaceutical products. Pharmaceutical and biotechnology firms rely on the company for radioisotopes and contract manufacturing, particularly for radiopharmaceutical development.
Research institutions and academic laboratories utilize Eckert & Ziegler's radioactive components for drug discovery and preclinical studies. Industrial clients depend on their isotope products for essential applications like industrial gauging, precise measurement, and environmental analysis. Government agencies and regulatory bodies are crucial stakeholders, influencing market dynamics through regulations and standards.
| Customer Segment | Key Needs | 2024 Market Relevance |
|---|---|---|
| Hospitals & Cancer Centers | Brachytherapy, Radiopharmaceuticals (diagnostic & therapeutic) | Driven by increasing cancer incidence; WHO projected over 28M cases annually by 2040. |
| Pharma & Biotech | Radioisotopes, Contract Manufacturing (radiopharma development) | Global radiopharmaceutical market valued at ~$7.5 billion in 2024, growing with targeted therapies. |
| Research Institutions | Radioactive components, Isotope services (drug discovery, preclinical) | Robust demand for specialized radioisotopes in preclinical research remained in 2024. |
| Industrial Clients | Isotope products (gauging, measurement, environmental analysis) | Steady demand in industrial gauging market in 2024 due to manufacturing efficiency needs. |
Cost Structure
Eckert & Ziegler's cost structure heavily relies on acquiring raw materials and the intricate, energy-demanding process of isotope production. This involves securing precursor materials for radioisotopes such as Lutetium-177 and Actinium-225, alongside the operational expenses for specialized facilities like cyclotrons.
For instance, the company's 2024 financial reports indicate that the cost of goods sold, which encompasses these raw material and production expenses, represented a substantial portion of their overall expenditure. Fluctuations in global commodity prices and energy rates directly influence the profitability of these critical operations.
Eckert & Ziegler's commitment to innovation fuels significant research and development (R&D) expenses. These costs are essential for developing novel isotope technologies, radiopharmaceuticals, and enhancing manufacturing processes. For instance, in 2023, the company reported R&D expenses of €27.9 million, highlighting the substantial investment in staying at the forefront of medical technology.
Manufacturing and operational costs for Eckert & Ziegler Strahlen- und Medizintechnik are significant, reflecting the specialized nature of their production. These include expenses for running advanced manufacturing facilities, rigorous quality control processes, and the upkeep of critical equipment. For instance, in 2023, the company reported a cost of sales of €221.2 million, underscoring the substantial investment in these operational areas.
The company also incurs costs related to maintaining its global network of contract manufacturing organization (CMO) services. This involves managing facilities and personnel across different regions to support their clients' needs. Furthermore, investments in expanding production capacity to meet growing market demand contribute to these overall manufacturing and operational expenditures.
Personnel and Expertise-Related Costs
Eckert & Ziegler Strahlen- und Medizintechnik incurs substantial costs related to its personnel, reflecting the highly specialized knowledge required in its field. Attracting, retaining, and developing a workforce of scientists, engineers, and technical experts is paramount for their operations, from cutting-edge research to stringent quality assurance and specialized service delivery.
These skilled individuals are the backbone of the company's innovation and operational excellence. In 2024, Eckert & Ziegler Strahlen- und Medizintechnik employed over 1,000 individuals, underscoring the significant investment in human capital necessary to maintain its competitive edge in the radiation technology and medical technology sectors.
- Highly Specialized Workforce: Costs associated with recruiting and maintaining a team of scientists, engineers, and technicians with expertise in radiation physics, medical device engineering, and related fields.
- Research and Development Investment: Significant expenditure on personnel dedicated to R&D, crucial for developing new products and improving existing technologies in a rapidly evolving market.
- Training and Development: Ongoing investment in training programs to ensure staff remain current with the latest scientific advancements, regulatory requirements, and technological innovations.
- Compensation and Benefits: Competitive salaries, bonuses, and benefits packages necessary to attract and retain top talent in a niche and demanding industry.
Sales, Marketing, and Distribution Expenses
Eckert & Ziegler Strahlen- und Medizintechnik's sales, marketing, and distribution expenses are vital for its global operations. These costs enable the company to connect with its diverse customer base and ensure timely product delivery across international markets.
Key expenditures include maintaining a direct sales force, actively participating in major industry conferences, and managing the complex logistics of transporting radioactive materials. These activities are fundamental to penetrating new markets and expanding customer reach.
- Sales Force: Costs associated with employing and training a specialized sales team to engage with healthcare professionals and research institutions.
- Marketing & Conferences: Investments in promotional materials, digital marketing campaigns, and participation in key global medical and radiation technology trade shows. For instance, in 2023, the company likely allocated significant resources to events like the SNMMI Annual Meeting, a crucial platform for showcasing their diagnostic and therapeutic products.
- Global Logistics: Expenses related to the secure and compliant transportation of radioactive isotopes and medical devices, requiring specialized handling and adherence to international regulations. This includes warehousing and distribution network maintenance.
- Supply Chain Management: Costs for building and maintaining a robust global supply chain to ensure product availability and reliability for customers worldwide.
Eckert & Ziegler's cost structure is dominated by the substantial expenses associated with raw material acquisition and the energy-intensive isotope production processes. These include the procurement of precursor materials for critical radioisotopes and the operational costs of specialized facilities.
Significant investment in research and development is a core component, driving innovation in isotope technologies and radiopharmaceuticals. The company also faces considerable manufacturing and operational costs, reflecting the specialized nature of its production and the upkeep of advanced facilities.
Personnel costs are high due to the need for a highly skilled workforce, including scientists and engineers. Sales, marketing, and global logistics, particularly for radioactive materials, also represent significant expenditures.
| Cost Category | Description | 2023 Data (Illustrative) |
|---|---|---|
| Cost of Sales | Raw materials, isotope production, manufacturing operations | €221.2 million |
| Research & Development | Personnel, equipment, and projects for new technologies | €27.9 million |
| Personnel Costs | Salaries, benefits for specialized workforce (over 1,000 employees in 2024) | Significant portion of operating expenses |
| Sales, Marketing & Distribution | Sales force, conferences, global logistics for radioactive materials | Essential for market penetration and customer reach |
Revenue Streams
Eckert & Ziegler's core revenue generation stems from the direct sales of radiopharmaceuticals and brachytherapy products. These are critical components in modern healthcare, with radiopharmaceuticals supporting both diagnostic imaging and therapeutic treatments in nuclear medicine, while brachytherapy products, like prostate seeds and ophthalmic applicators, are vital for localized cancer therapy.
The company experienced a notable surge in its Medical segment in 2024, largely fueled by robust demand for its radiopharmaceuticals. This increased sales activity highlights the growing importance and adoption of these specialized medical products within the healthcare industry.
Eckert & Ziegler's revenue streams are significantly bolstered by the sale of isotope products designed for industrial and scientific uses. These include both sealed and unsealed radiation sources, crucial for applications like gauging, precise measurement, detailed analysis, and instrument calibration.
This segment offers a vital diversification of income, extending beyond their core medical applications and demonstrating a broad market reach for their specialized products. The company reported a notable increase in sales within its Isotope Products segment during 2024, highlighting its growing importance.
Eckert & Ziegler generates revenue by offering specialized contract manufacturing and development services to radiopharmaceutical companies. This involves assisting these companies with the production and distribution of their investigational therapies.
The company also supplies essential radioisotopes crucial for the various stages of clinical development in the pharmaceutical sector. These services are underpinned by Eckert & Ziegler's advanced, dedicated facilities and deep technical knowledge, leading to revenue generation through customized contractual arrangements.
Licensing and Royalty Agreements
Eckert & Ziegler Strahlen- und Medizintechnik monetizes its intellectual property through licensing and royalty agreements. This involves granting access to its specialized isotope production methods and technologies. For instance, a significant license agreement was established with a Chinese joint venture concerning Ac-225, which includes an upfront payment and ongoing royalties.
These arrangements allow the company to generate revenue from its innovations without necessarily engaging in direct manufacturing for every application. This strategy is crucial for expanding the reach of their technologies globally and capturing value from their research and development investments.
- Licensing Fees: Eckert & Ziegler receives upfront payments for granting licenses to its proprietary technologies.
- Royalty Payments: The company earns ongoing revenue based on the sales or usage of products developed with its licensed technologies.
- Ac-225 Joint Venture: A specific agreement with a Chinese partner for Ac-225 production includes both a one-time payment and future royalties.
- Intellectual Property Monetization: This revenue stream directly leverages the company's R&D and patent portfolio.
Services for Radiation Protection and Analysis
Eckert & Ziegler also generates revenue by providing expert services in radiation protection and analysis. These offerings are crucial for clients needing to ensure safety and regulatory compliance when working with radioactive materials.
This service-based income stream is vital for the company's financial stability, offering a consistent revenue source beyond product sales. For instance, in 2024, the demand for specialized radiation safety consulting and material analysis remained robust across sectors like healthcare, research, and industry.
- Regulatory Compliance Assurance: Services help clients meet stringent safety standards.
- Expert Analysis: Providing specialized testing and assessment of radioactive materials.
- Safety Protocol Development: Assisting organizations in establishing and maintaining safe handling procedures.
- Ongoing Service Contracts: Securing recurring revenue through long-term support agreements.
Eckert & Ziegler's revenue streams are diverse, encompassing the direct sale of radiopharmaceuticals and brachytherapy products, crucial for medical diagnostics and cancer treatment. The company also generates income from isotope products used in industrial and scientific applications, providing a vital diversification beyond healthcare.
Furthermore, Eckert & Ziegler offers contract manufacturing and development services for radiopharmaceutical companies, leveraging its specialized facilities and expertise. Monetizing intellectual property through licensing, such as the Ac-225 joint venture, and providing radiation protection and analysis services contribute significantly to its financial performance.
| Revenue Stream | Primary Products/Services | 2024 Highlight |
|---|---|---|
| Radiopharmaceuticals & Brachytherapy | Diagnostic imaging agents, therapeutic isotopes, brachytherapy seeds | Robust demand driving Medical segment growth |
| Isotope Products | Sealed and unsealed radiation sources for industry and science | Notable sales increase in Isotope Products segment |
| Contract Manufacturing & Development | Production and distribution support for investigational therapies | Leveraging advanced facilities and technical knowledge |
| Licensing & Royalties | Access to isotope production methods and technologies | Ac-225 joint venture with upfront payment and royalties |
| Radiation Protection & Analysis Services | Safety consulting, material analysis, protocol development | Consistent demand across healthcare, research, and industry |
Business Model Canvas Data Sources
The Eckert & Ziegler Strahlen- und Medizintechnik Business Model Canvas is built upon comprehensive financial reports, detailed market research, and internal operational data. These sources ensure each component of the canvas is grounded in factual performance and strategic objectives.
