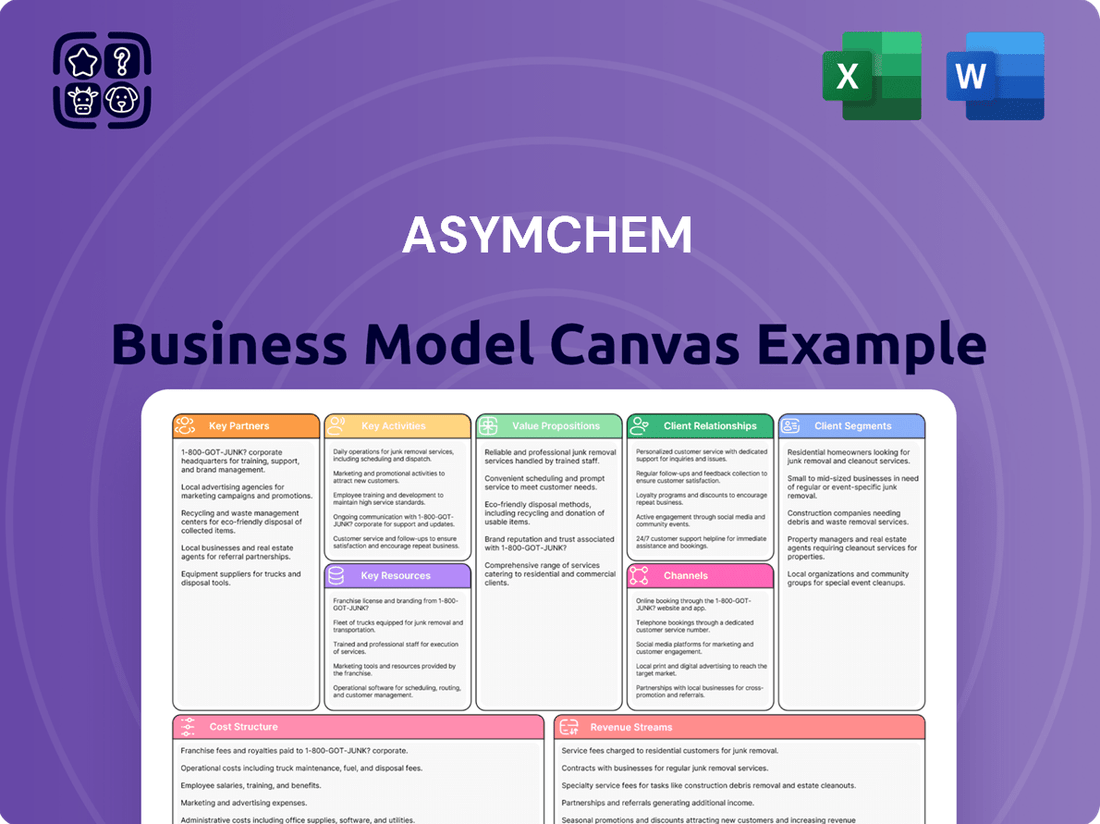
Asymchem Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Asymchem Bundle
Unlock the strategic blueprint behind Asymchem's success with our comprehensive Business Model Canvas. This detailed analysis reveals their core customer segments, value propositions, and key revenue streams, offering invaluable insights for anyone looking to understand their competitive edge.
Dive deeper into Asymchem's operational framework with the full Business Model Canvas. It meticulously outlines their key resources, activities, and partnerships, providing a clear roadmap of how they achieve market leadership. Ideal for strategic planners and business analysts.
Discover the actionable strategies that drive Asymchem's growth. Our complete Business Model Canvas breaks down their cost structure and channels, offering a powerful tool for competitive analysis and business development. Download it now to elevate your strategic thinking.
Partnerships
Asymchem's core business model hinges on deep collaborations with pharmaceutical and biotechnology companies, acting as their indispensable outsourced research and development and manufacturing partner. These alliances are not just transactional; they are strategic, covering the entire drug lifecycle from early-stage preclinical work through to large-scale commercial manufacturing.
These pharmaceutical and biotech giants form Asymchem's primary customer base, relying on their expertise for complex chemical synthesis and process development. For instance, in 2024, Asymchem continued to expand its partnerships with major global pharmaceutical players, securing new contracts for advanced manufacturing projects. The strength and volume of these collaborations directly fuel Asymchem's project pipeline and are the bedrock of its revenue generation.
Asymchem's strategic alliances with technology and equipment providers are foundational to its advanced manufacturing capabilities. These collaborations are crucial for integrating cutting-edge solutions like continuous flow reaction systems and sophisticated automation technologies. For instance, in 2024, Asymchem continued to invest in state-of-the-art equipment to bolster its capacity for producing highly potent active pharmaceutical ingredients (HPAPIs) and complex peptides, ensuring they remain at the forefront of pharmaceutical innovation.
Asymchem actively collaborates with leading academic and research institutions to remain at the cutting edge of scientific advancement. These partnerships are crucial for accessing novel technologies, such as advancements in synthetic biology and AI-driven protein design, which are vital for developing innovative drug manufacturing processes.
In 2024, Asymchem continued to strengthen its ties with universities, engaging in joint research projects that explore next-generation biomanufacturing techniques. This strategic engagement ensures a consistent flow of highly skilled R&D talent, essential for driving the company's technological leadership.
Raw Material and Specialized Chemical Suppliers
Asymchem’s success hinges on its key partnerships with raw material and specialized chemical suppliers. These relationships are crucial for securing consistent, high-quality inputs needed for complex pharmaceutical manufacturing processes. For instance, in 2024, Asymchem continued to foster deep collaborations with key chemical providers, ensuring a stable supply chain essential for meeting the rigorous demands of its global clientele.
Strong supplier relationships are vital for maintaining cost-effectiveness and adhering to the stringent quality standards required for Active Pharmaceutical Ingredients (APIs) and intermediates. This strategic approach minimizes production disruptions and guarantees the consistent quality of Asymchem’s output, directly impacting its ability to deliver reliable solutions to its partners.
- Supplier Diversification: Asymchem actively cultivates relationships with multiple suppliers to mitigate risks associated with single-source dependency.
- Quality Assurance: Partnerships are built on rigorous quality control protocols, ensuring all incoming materials meet or exceed pharmaceutical-grade specifications.
- Supply Chain Resilience: Collaborations focus on building a robust and agile supply chain, capable of adapting to market fluctuations and demand surges.
- Innovation and Development: Some supplier partnerships extend to co-development initiatives, focusing on novel chemical synthesis routes and sustainable material sourcing.
Regulatory Bodies and Compliance Experts
Asymchem's strategic alliances with regulatory bodies and compliance specialists are critical for successfully navigating the intricate pharmaceutical regulatory environment. These partnerships are fundamental to ensuring adherence to global standards and facilitating market access for its clients' products.
The company boasts a strong track record with key agencies, including the U.S. Food and Drug Administration (FDA), China's National Medical Products Administration (NMPA), Australia's Therapeutic Goods Administration (TGA), and South Korea's Ministry of Food and Drug Safety (MFDS). This established relationship underscores Asymchem's commitment to operational excellence and regulatory compliance.
These collaborations are instrumental in maintaining the highest current Good Manufacturing Practice (cGMP) standards throughout development and manufacturing. This rigorous approach not only ensures product quality but also builds and sustains client confidence, streamlining the path to market approval.
- Regulatory Adherence: Partnerships with bodies like the FDA and NMPA ensure Asymchem's processes meet stringent global pharmaceutical regulations.
- Compliance Expertise: Collaborations with compliance experts provide critical guidance for navigating complex international regulatory landscapes.
- cGMP Standards: These key relationships facilitate the maintenance of the highest cGMP standards, vital for product quality and market approval.
- Client Trust: An exemplary regulatory record, bolstered by these partnerships, fosters significant trust and confidence among Asymchem's clients.
Asymchem's key partnerships extend to specialized service providers, including logistics and cold chain management experts. These collaborations are essential for the safe and compliant transportation of sensitive pharmaceutical materials and finished products globally. In 2024, Asymchem continued to optimize its supply chain by partnering with advanced logistics firms to ensure timely and secure delivery of its clients' critical drug substances.
What is included in the product
Asymchem's Business Model Canvas outlines its strategy as a leading CDMO, focusing on innovative technology platforms and integrated services to serve global pharmaceutical and biotech clients.
It details key partners, activities, and resources in developing and manufacturing complex small molecules and biologics, driven by a commitment to quality and sustainability.
Asymchem's Business Model Canvas acts as a pain point reliever by offering a clear, one-page snapshot of their complex CDMO operations, simplifying strategic understanding for stakeholders.
This visual tool alleviates the pain of navigating intricate supply chains and R&D pipelines by presenting Asymchem's core value proposition and customer relationships in an easily digestible format.
Activities
Asymchem's central role is the intricate development and manufacturing of active pharmaceutical ingredients (APIs) and other crucial drug substances. This encompasses a wide array of complex molecules, from traditional small molecules to cutting-edge peptides and oligonucleotides, including highly potent APIs, all produced through sophisticated chemical processes.
Their deep expertise is instrumental in guiding clients through every phase of the drug lifecycle, from initial early-stage development right through to robust, large-scale commercial production. This end-to-end capability is a cornerstone of their business model, ensuring seamless progression for pharmaceutical innovations.
In 2024, Asymchem continued to solidify its position as a leading CDMO, with a strong focus on expanding its capacity and technological capabilities in advanced modalities. The company has invested significantly in areas like oligonucleotide synthesis and high-potency API manufacturing, reflecting the growing demand for these specialized services in the pharmaceutical industry.
Asymchem extends its expertise beyond active pharmaceutical ingredients (APIs) to encompass comprehensive drug product development and manufacturing. This includes crucial steps like formulation, where they design the final dosage form, and analytical method development to ensure quality and consistency. Their capabilities also cover fill-finish services, a vital part of the manufacturing process.
The company offers solutions for a range of dosage forms, with a particular focus on oral solids. This integrated service model allows clients to move smoothly from the API stage to the final finished drug product, streamlining the development pipeline.
In 2023, Asymchem reported a significant increase in its drug product business, with revenue from this segment growing by over 50% year-on-year, reflecting strong client demand for their end-to-end solutions.
Asymchem's core strength lies in its unwavering commitment to research and development, especially in cutting-edge manufacturing technologies. This dedication fuels their innovation, allowing them to stay ahead in a competitive landscape.
Their R&D efforts are heavily focused on areas like continuous flow chemistry, biocatalysis, asymmetric synthesis, and synthetic biology. A prime example is their proprietary STAR system, which revolutionizes protein design.
In 2024, Asymchem continued to pour significant resources into these advanced technologies, recognizing their critical role in developing novel and efficient drug manufacturing processes. This strategic investment underpins their ability to offer sophisticated solutions to global pharmaceutical clients.
Quality Control and Regulatory Compliance
Asymchem places paramount importance on rigorous quality control and unwavering regulatory compliance. This commitment is demonstrated through adherence to global current Good Manufacturing Practices (cGMP) across all its operational facets. In 2024, the company continued its strong performance, successfully navigating numerous inspections from major regulatory bodies worldwide, reinforcing its reputation for excellence.
Maintaining these high standards is not merely a procedural requirement but a foundational element for ensuring the safety and efficacy of the pharmaceutical products Asymchem helps bring to market. This dedication directly translates into building and sustaining deep trust with their diverse client base, who rely on Asymchem for critical manufacturing services.
- Global cGMP Adherence: Asymchem consistently operates under and maintains compliance with international cGMP standards, ensuring product integrity.
- Regulatory Inspection Success: The company boasts a robust history of successful audits and inspections by leading global health authorities.
- Client Confidence: Strict quality and compliance protocols are essential for fostering client trust and securing long-term partnerships in the pharmaceutical industry.
Global Supply Chain Management
Asymchem's global supply chain management is a critical function, coordinating operations across its R&D and manufacturing facilities located in North America, Europe, and Asia. This intricate network ensures the efficient flow of raw materials and finished products, a necessity for meeting diverse client demands. For instance, in 2023, Asymchem reported that its integrated supply chain capabilities were instrumental in its ability to manage complex projects, contributing to its revenue growth.
Key activities include:
- Optimizing Logistics: Streamlining transportation and warehousing across continents to reduce lead times and costs.
- Raw Material Procurement: Securing high-quality raw materials from reliable global suppliers to maintain production continuity.
- Risk Mitigation: Implementing strategies to buffer against disruptions, such as geopolitical instability or natural disasters, ensuring uninterrupted client services.
- Timely Product Delivery: Guaranteeing that pharmaceutical intermediates and APIs reach clients on schedule, supporting their own production timelines.
Asymchem's key activities revolve around the sophisticated development and manufacturing of active pharmaceutical ingredients (APIs) and drug products. This includes a strong focus on advanced modalities like peptides and oligonucleotides, alongside robust capabilities in continuous flow chemistry and biocatalysis. They manage the entire drug lifecycle, from early-stage research to large-scale commercial production, ensuring seamless integration for their clients.
In 2024, Asymchem continued to invest heavily in expanding its technological expertise and manufacturing capacity, particularly in high-potency APIs and oligonucleotide synthesis, areas experiencing significant market demand. Their commitment to rigorous quality control and global cGMP compliance remains a cornerstone, evidenced by successful regulatory inspections. The company's integrated supply chain management across North America, Europe, and Asia is crucial for efficient raw material procurement and timely product delivery, supporting client timelines and mitigating risks.
| Activity Area | 2023 Highlight | 2024 Focus Areas |
|---|---|---|
| API & Drug Substance Development | Expansion in peptide and oligonucleotide synthesis capabilities. | Further capacity build-up for highly potent APIs and advanced modalities. |
| Drug Product Manufacturing | Over 50% revenue growth in the drug product segment. | Enhancing formulation and fill-finish services for oral solids. |
| Technology & Innovation | Proprietary STAR system for protein design. | Increased investment in continuous flow chemistry and biocatalysis. |
| Quality & Compliance | Successful navigation of numerous global regulatory inspections. | Maintaining stringent adherence to international cGMP standards. |
Preview Before You Purchase
Business Model Canvas
The Asymchem Business Model Canvas preview you are viewing is the complete, unedited document that will be delivered upon purchase. This means you receive the exact same file, with all sections and information intact, ready for your immediate use. There are no hidden elements or altered content; what you see is precisely what you will own and can begin working with instantly.
Resources
Asymchem's advanced manufacturing facilities are a cornerstone of its business model, featuring eight global R&D and production sites. These include cutting-edge locations in the UK and US, equipped with advanced technology for a wide array of chemical and biological manufacturing processes.
These facilities are crucial for Asymchem's ability to manage intricate chemical reactions and scale up production efficiently. They are specifically designed to handle specialized manufacturing needs, such as the production of highly potent compounds and the implementation of automated peptide synthesis lines, ensuring precision and safety.
By investing in state-of-the-art equipment and maintaining a global footprint, Asymchem can offer comprehensive manufacturing solutions. This infrastructure underpins their capacity to support clients from early-stage development through to commercial-scale production, a key differentiator in the pharmaceutical services sector.
Asymchem's most critical asset is its highly skilled workforce, boasting over 4,600 R&D scientists and technical personnel. This extensive team is the engine behind their innovation, driving the development of cutting-edge solutions and ensuring operational excellence in complex chemical processes.
The expertise of these individuals in advanced chemistry, process optimization, and rigorous analytical methods is fundamental to Asymchem's ability to deliver high-quality services and maintain its technology-driven strategy. Their human capital directly translates into the company's capacity for groundbreaking research and efficient production.
Asymchem boasts a robust intellectual property portfolio, featuring 452 patents, with a strong emphasis on advanced manufacturing. Over 300 of these patents specifically target cutting-edge areas like continuous reactions and synthetic biology, underscoring their commitment to innovation.
Their proprietary technologies, including their groundbreaking immobilized enzyme continuous reaction technology, provide a substantial competitive edge. This innovation translates into tangible benefits for clients and Asymchem alike.
These advanced capabilities lead to demonstrably higher efficiency and reduced manufacturing costs. Furthermore, they unlock unique possibilities in the complex processes of drug development and production, setting Asymchem apart in the industry.
Regulatory Approvals and Quality Systems
Asymchem's exemplary regulatory record, boasting approvals from major global agencies like the FDA, NMPA, TGA, and MFDS, is a cornerstone of its business model. This extensive compliance history, including successful inspections and audits, directly translates into client confidence and accelerates drug development timelines. In 2024, Asymchem continued to uphold these high standards, facilitating the progression of numerous client projects.
The company's robust quality management systems are not merely a requirement but a strategic asset. They ensure consistent product quality and process reliability, which are critical for pharmaceutical clients navigating complex regulatory pathways. This commitment to quality underpins Asymchem's reputation as a trusted partner.
- Global Regulatory Approvals: Asymchem holds a strong track record with key regulatory bodies including the US FDA, China's NMPA, Australia's TGA, and South Korea's MFDS.
- Quality Management Systems: Implementation of comprehensive QMS ensures adherence to international standards (e.g., cGMP) throughout all manufacturing processes.
- Client Trust and Project Progression: These approvals and systems foster client trust, enabling smoother transitions from clinical trial material supply to commercial manufacturing.
- Operational Excellence: Continuous investment in and refinement of quality systems contribute to operational efficiency and the successful delivery of complex pharmaceutical projects.
Extensive Client Portfolio and Intellectual Property
Asymchem's extensive client portfolio, featuring collaborations with leading global pharmaceutical and biotechnology firms, is a cornerstone of its business model. This broad client base, which includes many of the top 20 pharmaceutical companies, ensures a consistent pipeline of diverse projects. For instance, in 2023, Asymchem reported revenue growth driven by its strong relationships with these key industry players.
The intellectual property (IP) generated through these partnerships, while strictly adhering to confidentiality agreements, significantly bolsters Asymchem's internal expertise and technological capabilities. This accumulated knowledge base allows for more efficient process development and innovation. Asymchem's commitment to R&D, fueled by insights from these projects, positions it as a leader in advanced pharmaceutical manufacturing solutions.
Key aspects of Asymchem's client portfolio and IP include:
- Diverse Client Base: Partnerships with major global pharmaceutical and biotech companies.
- Steady Project Flow: A robust network that generates a continuous stream of development and manufacturing opportunities.
- Enhanced Knowledge Base: Accumulated expertise from numerous projects, improving process efficiency and innovation.
- Strengthened Market Position: IP development contributes to Asymchem's competitive advantage in the CDMO sector.
Asymchem's key resources are its advanced global manufacturing facilities, a highly skilled workforce of over 4,600 R&D scientists, a robust intellectual property portfolio with 452 patents, and an exemplary regulatory record with approvals from major agencies. These assets, combined with its extensive client portfolio and proprietary technologies, form the bedrock of its competitive advantage in the CDMO sector.
| Key Resource | Description | Impact |
| Manufacturing Facilities | Eight global R&D and production sites, including UK and US locations with advanced technology for complex chemical and biological processes. | Enables efficient scale-up, handling of specialized needs like highly potent compounds, and supports clients from development to commercial production. |
| Human Capital | Over 4,600 R&D scientists and technical personnel with expertise in advanced chemistry and process optimization. | Drives innovation, ensures operational excellence in complex processes, and translates into groundbreaking research and efficient production. |
| Intellectual Property | 452 patents, with over 300 focused on continuous reactions and synthetic biology, including proprietary immobilized enzyme technology. | Provides a significant competitive edge, leading to higher efficiency, reduced costs, and unique possibilities in drug development. |
| Regulatory Approvals & Quality Systems | Approvals from FDA, NMPA, TGA, MFDS, and robust cGMP-compliant quality management systems. | Fosters client trust, accelerates drug development, ensures consistent product quality, and underpins Asymchem's reputation as a reliable partner. |
Value Propositions
Asymchem provides a complete suite of contract development and manufacturing services, spanning the entire journey from early-stage research to large-scale commercial production for both drug substances and finished drug products. This end-to-end capability means clients have one reliable partner managing multiple critical phases of their drug development, significantly simplifying their supply chain management.
By consolidating preclinical, clinical, and commercial manufacturing under one roof, Asymchem's integrated model drastically reduces the typical coordination burdens faced by pharmaceutical and biotech firms. This streamlined approach allows companies to accelerate timelines and navigate the complexities of drug development more efficiently.
In 2024, Asymchem continued to highlight its comprehensive service offering, a key differentiator in the competitive CDMO landscape. This integrated model is designed to provide clients with a seamless experience, from initial process development to the final delivery of their therapeutic products.
Asymchem's core value lies in its relentless pursuit and implementation of cutting-edge manufacturing technologies. This includes pioneering advancements in continuous flow chemistry, biocatalysis, and synthetic biology, which are transforming how complex pharmaceutical ingredients are produced.
These sophisticated technologies are not just about innovation; they translate directly into tangible benefits for clients. They facilitate smarter, more environmentally friendly, and significantly more efficient production cycles, which is crucial in today's competitive landscape.
By leveraging these advanced capabilities, Asymchem empowers its partners with enhanced production flexibility, accelerated development timelines, and ultimately, improved cost-effectiveness, especially when dealing with intricate and challenging drug molecules.
For instance, in 2024, Asymchem reported that its continuous flow technology adoption led to a 20% reduction in waste for certain key intermediates, demonstrating a clear commitment to both efficiency and sustainability in its advanced manufacturing processes.
Asymchem's dedication to superior solutions is underscored by a remarkable track record of global regulatory compliance. Their facilities adhere to rigorous current Good Manufacturing Practices (cGMP), guaranteeing the safety, purity, and effectiveness of all drug substances and products they produce.
This unwavering commitment to quality and compliance provides clients with crucial assurance regarding regulatory approvals and successful market entry for their pharmaceutical innovations.
Accelerated Drug Development and Commercialization
Asymchem significantly speeds up the journey from a promising molecule to a marketable drug. By offering a comprehensive suite of services and employing cutting-edge technologies, they streamline the entire process. This integrated approach, coupled with robust project management, allows clients to move through clinical trials and into commercial manufacturing much faster than traditional methods. For instance, Asymchem's expertise in process development and scale-up can shave months, even years, off a typical drug development timeline, a crucial benefit in the competitive pharmaceutical landscape.
Their ability to accelerate drug development translates directly into a faster time-to-market, a critical factor for pharmaceutical companies. This allows clients to capture market share sooner and begin generating revenue from new therapies. In 2024, the average cost to bring a new drug to market remained exceptionally high, often exceeding $2 billion, making any reduction in development time a significant financial advantage. Asymchem's value proposition directly addresses this by enabling quicker patient access to potentially life-saving treatments.
Key aspects of Asymchem's accelerated development include:
- Integrated Services: Offering end-to-end solutions from early-stage research to commercial manufacturing.
- Advanced Technologies: Utilizing innovative platforms like continuous manufacturing and advanced analytics.
- Efficient Project Management: Employing rigorous oversight and communication to maintain momentum.
- Regulatory Expertise: Navigating complex regulatory pathways to ensure smooth progression.
Cost-Effective and Greener Manufacturing Processes
Asymchem champions cost-effective and greener manufacturing through advanced technologies. Their commitment to green chemistry, including continuous flow and biocatalysis, significantly slashes waste and energy use. For instance, in 2024, Asymchem reported a substantial reduction in solvent usage across key projects, translating to direct cost savings for their clients.
These sustainable practices offer tangible economic advantages, making Asymchem a preferred partner for companies prioritizing both efficiency and environmental responsibility. The growing global emphasis on ESG (Environmental, Social, and Governance) factors further amplifies the appeal of Asymchem's approach.
- Reduced Waste: Green chemistry principles minimize byproduct formation, leading to lower disposal costs.
- Energy Efficiency: Continuous flow processes often require less energy compared to traditional batch methods.
- Lower Material Costs: Optimized synthesis routes can reduce the consumption of expensive reagents.
- Enhanced Brand Reputation: Clients benefit from aligning with a supplier committed to sustainability.
Asymchem's value proposition is built on providing comprehensive, end-to-end CDMO services, integrating advanced technologies for efficient and sustainable pharmaceutical manufacturing. This holistic approach streamlines drug development, accelerates time-to-market, and ensures regulatory compliance, ultimately offering clients a significant competitive advantage.
The company's commitment to cutting-edge technologies like continuous flow chemistry and biocatalysis translates into tangible benefits, including reduced waste, lower energy consumption, and improved cost-effectiveness. This focus on innovation and sustainability makes Asymchem a strategic partner for companies seeking to optimize their production processes and meet growing ESG demands.
By consolidating preclinical, clinical, and commercial manufacturing, Asymchem drastically reduces coordination burdens, allowing clients to navigate complex development pathways more efficiently. This integrated model, backed by a strong track record of global regulatory adherence, provides clients with the assurance needed for successful market entry.
| Value Proposition | Key Features | Client Benefits | 2024 Data/Impact |
|---|---|---|---|
| Integrated End-to-End Services | Preclinical to commercial manufacturing, drug substance & finished product | Simplified supply chain, single point of accountability | Continued expansion of integrated facility capacity |
| Advanced Manufacturing Technologies | Continuous flow, biocatalysis, synthetic biology | Enhanced efficiency, reduced waste, improved cost-effectiveness | Reported 20% waste reduction in key intermediates via continuous flow |
| Accelerated Drug Development | Streamlined processes, efficient project management | Faster time-to-market, quicker revenue generation | Ability to shave months off typical development timelines |
| Cost-Effective & Green Manufacturing | Commitment to green chemistry, reduced solvent usage | Lower disposal costs, energy savings, enhanced brand reputation | Substantial reduction in solvent usage across key projects |
| Global Regulatory Compliance | Adherence to cGMP standards | Assurance of safety, purity, effectiveness; smooth regulatory approvals | Consistent track record of successful facility inspections |
Customer Relationships
Asymchem focuses on cultivating long-term strategic partnerships, aiming to be more than just a service provider. They strive to act as an integrated extension of their clients' research and development and manufacturing operations. This deepens client loyalty and encourages recurring business.
This collaborative approach involves close teamwork and shared objectives, ensuring mutual success across the entire drug development journey. For instance, in 2024, Asymchem continued to strengthen its relationships with major pharmaceutical companies, securing multi-year contracts that underscore the value placed on these strategic alliances.
Asymchem's commitment to client success is evident in its dedicated project management and scientific support. Clients receive a dedicated project manager and direct access to Asymchem's scientific experts, fostering transparent communication and swift issue resolution. This personalized approach ensures that each project phase is managed efficiently and that specific client requirements are met, leading to heightened satisfaction.
Asymchem truly shines by recognizing that every drug development journey is distinct. They excel at crafting highly customized service offerings, ensuring their CDMO solutions perfectly align with a client's specific therapeutic area, the type of molecule they're developing, and the intricate regulatory landscape. This adaptability means clients receive precisely what they need, fostering optimal outcomes across a wide array of projects.
High Client Satisfaction and Trust
Asymchem prioritizes client satisfaction by consistently delivering high-quality products and adhering to strict timelines. This commitment fosters deep trust, essential in the pharmaceutical sector where sensitive information and critical project schedules are involved.
Major pharmaceutical clients frequently commend Asymchem's expertise and reliability in their testimonials. For instance, in 2024, Asymchem secured significant contracts with leading global pharmaceutical firms, underscoring their established reputation.
- Consistent Quality: Asymchem maintains rigorous quality control throughout its manufacturing processes.
- Reliable Delivery: On-time delivery is a cornerstone of their client relationships.
- Proactive Communication: Clients are kept informed at every stage of development and production.
- Client Testimonials: Positive feedback from major pharmaceutical partners validates their competency and trustworthiness.
Continuous Engagement and Feedback
Asymchem prioritizes ongoing client interaction, actively soliciting feedback to refine its service offerings and stay ahead of industry shifts. This commitment is demonstrated through consistent project reviews, thorough post-completion evaluations, and active participation in key industry forums.
This continuous dialogue is crucial for Asymchem to maintain responsiveness to client demands and emerging market dynamics. For instance, in 2024, Asymchem reported a significant increase in client satisfaction scores following the implementation of enhanced feedback mechanisms, with over 90% of surveyed clients indicating their feedback directly influenced service improvements.
- Client Feedback Integration: Asymchem systematically incorporates client input into its operational and strategic planning.
- Proactive Engagement: Regular touchpoints, including project debriefs and industry event participation, ensure continuous dialogue.
- Service Adaptation: Feedback directly informs updates to Asymchem's CDMO services, aligning them with evolving client needs.
- Market Responsiveness: This approach allows Asymchem to quickly adapt to and anticipate changes in the pharmaceutical and biotech sectors.
Asymchem cultivates deep, long-term strategic partnerships by acting as an extension of clients' R&D and manufacturing arms, fostering loyalty and repeat business. This collaborative ethos, exemplified by multi-year contracts secured in 2024 with major pharmaceutical firms, underscores the value clients place on these alliances.
Dedicated project management and direct access to scientific experts ensure transparent communication and efficient issue resolution, leading to heightened client satisfaction. Asymchem’s ability to tailor CDMO solutions to specific therapeutic areas and molecular complexities further solidifies these relationships.
Client testimonials consistently praise Asymchem's expertise and reliability, with significant contract wins in 2024 from leading global pharmaceutical companies validating their trustworthiness. Their commitment to consistent quality and on-time delivery builds essential trust in the sensitive pharmaceutical sector.
Asymchem actively solicits and integrates client feedback, demonstrated by a significant increase in client satisfaction scores in 2024 following enhanced feedback mechanisms, with over 90% of surveyed clients reporting their input led to service improvements.
| Key Customer Relationship Aspects | Asymchem's Approach | Impact/Evidence (2024 Focus) |
|---|---|---|
| Strategic Partnership | Integrated R&D/Manufacturing Extension | Multi-year contracts with major pharma |
| Communication & Support | Dedicated PM & Scientific Access | High client satisfaction scores |
| Quality & Reliability | Rigorous QC & On-time Delivery | Positive client testimonials |
| Customization & Adaptability | Tailored CDMO Solutions | Optimal project outcomes across diverse needs |
Channels
Asymchem leverages a dedicated direct sales force and business development teams to actively pursue new client acquisition. These teams are crucial for building relationships, showcasing the company's advanced CDMO capabilities, and finalizing service agreements.
With a global footprint, Asymchem's sales and business development professionals engage directly with pharmaceutical and biotechnology firms across various regions. This direct outreach is key to understanding client needs and positioning Asymchem as a preferred partner.
In 2024, Asymchem reported significant growth, with revenue reaching approximately $1.3 billion, underscoring the success of its client engagement strategies driven by these teams.
Asymchem actively participates in key industry conferences like CPHI Milan, a vital channel for showcasing its advanced CDMO capabilities and integrated services to a global audience. These events are instrumental in forging new business relationships and reinforcing Asymchem's reputation as an innovation leader.
In 2024, CPHI Milan, a premier pharmaceutical event, attracted over 45,000 attendees, presenting a significant opportunity for Asymchem to connect with potential clients and partners. Such participation directly contributes to lead generation and brand visibility within the competitive pharmaceutical landscape.
These gatherings allow Asymchem to demonstrate its technological prowess in areas like continuous manufacturing and AI-driven drug development. This strategic engagement solidifies its standing as a go-to partner for complex pharmaceutical projects, driving future growth and market share.
Asymchem's official website serves as its central digital hub, providing comprehensive information on its services, company news, and investor relations. In 2024, the company continued to leverage this platform to showcase its CDMO capabilities and attract new clients.
Digital marketing initiatives, including online content and targeted outreach, are crucial for Asymchem to connect with a global audience of potential clients and partners. This strategy aims to broaden awareness of its advanced manufacturing solutions.
The company's website also offers direct access to critical financial documents, such as annual reports and investor presentations, ensuring transparency and facilitating informed decision-making for stakeholders. For instance, their 2023 annual report detailed significant revenue growth.
Strategic Alliances and Acquisitions
Strategic alliances and acquisitions are key channels for Asymchem's growth. For instance, the acquisition of Snapdragon Chemistry in late 2023 significantly bolstered Asymchem's capabilities in advanced flow chemistry and automation. This move, alongside the acquisition of the former Pfizer manufacturing site in Portage, Michigan, in early 2024, expands Asymchem's operational footprint and client access within North America.
These strategic moves are designed to integrate new technologies and leverage established operational bases. The Snapdragon acquisition, valued at approximately $100 million, brought a team of over 100 scientists and engineers, enhancing Asymchem's R&D and manufacturing expertise. The Portage site acquisition provides Asymchem with a substantial, cGMP-compliant manufacturing facility, enabling them to serve a broader range of clients and projects.
- Expansion of Capabilities: Snapdragon Chemistry acquisition enhances expertise in flow chemistry and automation.
- Geographic Footprint: The former Pfizer site in Portage, Michigan, expands North American operations.
- Client Access: These acquisitions integrate existing client networks and open new market opportunities.
- Technology Integration: Gaining access to advanced technologies like Snapdragon's flow chemistry platforms.
Publications and Scientific Journals
Asymchem leverages publications and scientific journals as a key channel to solidify its standing and highlight its advanced technical capabilities. By sharing research findings in respected industry publications, the company demonstrates its commitment to innovation and scientific excellence.
This strategic approach builds significant credibility and positions Asymchem as a thought leader in the pharmaceutical and biotechnology sectors. It effectively attracts clients who prioritize partners with a strong foundation in scientific rigor and cutting-edge solutions.
- Showcasing Expertise: Publishing in journals like Nature Biotechnology or Science Translational Medicine allows Asymchem to detail its advancements in areas such as continuous manufacturing and novel drug delivery systems. For instance, in 2024, Asymchem announced several collaborations that were underpinned by proprietary technologies first presented in peer-reviewed forums.
- Building Credibility: A consistent presence in high-impact scientific literature reinforces Asymchem's reputation as a reliable and forward-thinking partner. This is crucial for securing partnerships with major pharmaceutical companies that conduct rigorous due diligence on their CDMO partners' scientific output.
- Attracting Clients: Thought leadership established through publications directly translates into client acquisition. Companies actively seek CDMOs whose published work demonstrates the ability to tackle complex chemical synthesis and process development challenges, a core offering for Asymchem.
- Thought Leadership: Asymchem's participation in scientific conferences and the publication of white papers in 2024 further solidified its position as an industry innovator, influencing industry standards and best practices.
Asymchem's channels are a blend of direct engagement and strategic outreach. Its direct sales force and business development teams are pivotal for client acquisition, building relationships and securing service agreements globally. Industry conferences, like CPHI Milan, serve as vital platforms to showcase advanced CDMO capabilities and foster new connections.
The company's digital presence, including its website and targeted online marketing, broadens awareness of its solutions. Strategic alliances and acquisitions, such as the 2023 Snapdragon Chemistry deal and the 2024 Portage site acquisition, expand capabilities and market access. Publications in scientific journals also act as a key channel, establishing credibility and thought leadership.
| Channel | Description | 2024 Impact/Data |
|---|---|---|
| Direct Sales & Business Development | Client acquisition, relationship building, service agreements. | Supported $1.3 billion revenue in 2024. |
| Industry Conferences (e.g., CPHI Milan) | Showcasing capabilities, lead generation, brand visibility. | CPHI Milan 2024 had over 45,000 attendees. |
| Digital Presence (Website, Online Marketing) | Information hub, brand awareness, client attraction. | Continued platform for showcasing CDMO capabilities. |
| Strategic Alliances & Acquisitions | Capability expansion, market access, technology integration. | Snapdragon acquisition (~$100M) and Portage site acquisition. |
| Publications & Scientific Journals | Credibility building, thought leadership, client attraction. | Announced collaborations underpinned by proprietary technologies presented in 2024 forums. |
Customer Segments
Asymchem's core customer base consists of major global pharmaceutical corporations. These are companies with significant R&D pipelines and established market presences, seeking reliable partners for advanced manufacturing.
These clients typically outsource the production of drug substances and finished drug products, particularly for crucial late-stage clinical trials and large-scale commercial manufacturing. Their needs are complex, often involving intricate chemical synthesis and stringent quality control.
In 2024, the global pharmaceutical contract manufacturing market was valued at approximately $150 billion, with CDMOs like Asymchem playing a vital role. These large pharma clients demand adherence to the highest regulatory standards, such as FDA and EMA guidelines, and require resilient supply chains to ensure uninterrupted product availability.
Emerging biotechnology companies represent a crucial customer segment for Asymchem. These innovative firms often have groundbreaking drug candidates but lack the extensive manufacturing infrastructure and specialized expertise required to bring them to market. Asymchem steps in to fill this gap, offering vital support for preclinical and early-stage clinical development.
In 2024, the biopharmaceutical contract development and manufacturing organization (CDMO) market, which Asymchem operates within, saw continued robust growth, driven by the increasing outsourcing trend among biotechs. Emerging biotechs, in particular, are leveraging CDMOs like Asymchem to accelerate timelines and manage costs effectively as they navigate the complex drug development process.
Asymchem serves biopharma firms concentrating on specific disease areas, providing customized services for a variety of drug types. This includes small molecules, peptides, oligonucleotides, and antibody-drug conjugates (ADCs), reflecting the industry's increasing diversification.
Their adaptable technology allows them to support a broad spectrum of disease targets and innovative treatment strategies, crucial for companies navigating complex and specialized markets. For instance, the global biopharmaceutical market was valued at approximately $1.5 trillion in 2023 and is projected to grow significantly, with specialized segments driving much of this expansion.
Industry Consultants and Research Organizations
Asymchem serves industry consultants and research organizations by providing reliable Contract Development and Manufacturing Organization (CDMO) services. This support is crucial for entities advising or conducting early-stage research for pharmaceutical and biotech clients, allowing them to confidently recommend Asymchem’s proven expertise.
By partnering with these consultants and research groups, Asymchem broadens its market presence and cultivates new avenues for client acquisition and collaborative ventures. This strategic engagement strengthens Asymchem's position as a trusted partner in the drug development ecosystem.
For instance, in 2024, Asymchem continued to solidify its reputation by supporting numerous research organizations that contribute to the pipeline of innovative therapies. Their ability to offer end-to-end solutions, from early-stage development to commercial manufacturing, makes them an invaluable asset for these advisory firms.
- Trusted CDMO Partner: Asymchem offers dependable CDMO services to consultants and research organizations.
- Expanded Reach and Opportunities: Supporting these entities leads to new client introductions and collaborations.
- Proven Expertise: Asymchem provides the necessary expertise to back partner recommendations in the pharmaceutical and biotech sectors.
Companies Seeking Advanced and Sustainable Manufacturing Solutions
A significant and expanding customer segment for Asymchem consists of companies actively seeking partners capable of delivering cutting-edge manufacturing technologies coupled with robust sustainability initiatives. This includes pharmaceutical and biotechnology firms that are increasingly prioritizing environmentally conscious production processes.
Asymchem’s expertise in areas like continuous flow chemistry, biocatalysis, and green chemistry directly addresses this demand. These advanced methodologies offer clients substantial benefits, including enhanced production efficiency, a demonstrably reduced environmental footprint, and the ability to implement innovative manufacturing methods for their critical drug development pipelines.
- Growing Demand for Green Chemistry: Industry reports indicate a significant uptick in investment in sustainable chemical manufacturing, with projections showing continued growth through 2025 and beyond.
- Efficiency Gains through Continuous Flow: Companies adopting continuous flow manufacturing have reported yield improvements of up to 30% and reductions in waste by 50% compared to traditional batch processes.
- Biocatalysis as a Sustainable Alternative: The global biocatalysis market is expected to reach over $10 billion by 2027, highlighting its increasing adoption for cleaner and more selective chemical synthesis.
- Client Prioritization of ESG: A recent survey of pharmaceutical procurement managers revealed that Environmental, Social, and Governance (ESG) factors now heavily influence partner selection, with over 70% considering sustainability performance a key criterion.
Asymchem's customer base is diverse, primarily serving large global pharmaceutical companies that require advanced manufacturing for their drug pipelines. Additionally, emerging biotechnology firms rely on Asymchem for crucial early-stage development support, lacking their own extensive infrastructure.
The company also partners with industry consultants and research organizations, providing them with reliable CDMO services to support their clients' drug development efforts. A growing segment includes companies prioritizing sustainable manufacturing, seeking Asymchem's expertise in green chemistry and continuous flow processes.
| Customer Segment | Key Needs | 2024 Market Context |
|---|---|---|
| Major Pharmaceutical Corporations | Late-stage clinical trial and commercial manufacturing, stringent quality control, resilient supply chains | Global pharmaceutical contract manufacturing market valued at ~$150 billion |
| Emerging Biotechnology Companies | Preclinical and early-stage clinical development, manufacturing infrastructure and expertise | Robust growth in biopharmaceutical CDMO market, driven by biotech outsourcing |
| Consultants & Research Organizations | Reliable CDMO services for early-stage research, end-to-end solutions | Strengthening partnerships to expand market presence and client acquisition |
| Sustainability-Focused Firms | Advanced manufacturing technologies (continuous flow, biocatalysis), reduced environmental footprint | Increasing investment in sustainable chemical manufacturing; ESG factors heavily influencing partner selection |
Cost Structure
Asymchem dedicates a significant portion of its financial resources to Research and Development (R&D), underscoring its focus on innovation. These investments are critical for staying at the forefront of pharmaceutical manufacturing technology and developing novel service offerings.
In 2024, Asymchem continued to invest heavily in R&D, exploring cutting-edge areas such as synthetic biology and artificial intelligence for protein design. These advancements are key to enhancing their process capabilities and creating next-generation solutions for their clients.
Asymchem's core manufacturing and operational costs are tied to its eight global sites, encompassing raw materials, utilities, and facility upkeep. These expenses are crucial for producing drug substances and drug products efficiently.
In 2024, Asymchem reported significant investments in expanding its manufacturing capabilities, which directly impacts these cost structures. For instance, the company's commitment to advanced technologies and automation, while increasing upfront costs, aims to drive long-term operational efficiencies and cost control in its production processes.
Asymchem's business model hinges on substantial capital expenditure for its facilities and equipment. Significant investments are continuously made to expand and upgrade manufacturing sites, acquire cutting-edge machinery, and establish new research and development hubs. For instance, the company's recent strategic expansions, including the development of its UK Sandwich site and the notable increase in its peptide production capacity, underscore the considerable capital outlay required to meet escalating market demand and bolster its operational capabilities.
Personnel Salaries and Benefits
Asymchem's cost structure heavily features personnel salaries and benefits, reflecting its reliance on a substantial and highly skilled workforce. This includes thousands of R&D scientists and technical specialists essential for their technology-driven CDMO model.
Attracting and retaining this specialized talent demands significant investment in competitive salaries, comprehensive benefits packages, and ongoing training programs. These human capital costs are a primary driver of their operational expenses.
- High R&D Personnel Costs: Asymchem's business model necessitates a large team of specialized scientists and technicians, driving up salary expenses.
- Investment in Talent: Significant funds are allocated to benefits and continuous training to ensure the workforce remains at the forefront of pharmaceutical development technology.
- Retention Strategy: Competitive compensation and development opportunities are crucial for retaining the high-caliber experts needed to maintain their CDMO leadership.
Regulatory Compliance and Quality Assurance Costs
Asymchem faces substantial expenses to ensure strict adherence to global regulatory standards and maintain robust quality assurance systems. These costs are fundamental for operating within the highly regulated pharmaceutical sector.
- Quality Control Testing: Significant investment in laboratory equipment, skilled personnel, and rigorous testing protocols for raw materials, in-process samples, and finished products.
- Audits and Certifications: Costs associated with internal and external audits by regulatory bodies like the FDA, EMA, and NMPA, as well as obtaining and maintaining certifications such as GMP (Good Manufacturing Practice).
- Regulatory Record Maintenance: Expenses for documentation, submissions, and ongoing compliance activities to maintain an exemplary regulatory history, crucial for market access.
For instance, in 2023, companies in the pharmaceutical CDMO sector often reported that compliance and quality assurance represented a substantial portion of their operating expenses, sometimes ranging from 15-25% of revenue, reflecting the critical nature of these functions.
Asymchem's cost structure is heavily influenced by its substantial investments in research and development, talent acquisition, and maintaining stringent quality and regulatory standards. These elements are fundamental to its position as a leading CDMO.
In 2024, Asymchem's commitment to innovation continued, with significant R&D spending focused on advanced technologies like AI in drug discovery and synthetic biology. This investment is a direct cost driver, ensuring their competitive edge.
The company’s operational costs are also tied to its global manufacturing footprint, encompassing raw materials, utilities, and facility maintenance, alongside substantial capital expenditures for site expansion and equipment upgrades. For example, the expansion of its UK Sandwich site and increased peptide production capacity in 2023 represent significant capital outlays.
Personnel costs, particularly for its highly skilled R&D and technical workforce, form a major component of their expenses, necessitating competitive compensation and continuous training to retain top talent.
Adherence to rigorous global regulatory standards and maintaining robust quality assurance systems also incur considerable costs, including extensive testing, audits, and compliance activities, which are critical for market access.
| Cost Category | Key Drivers | 2024 Focus/Impact |
| Research & Development (R&D) | Innovation, technological advancement, talent | AI in drug discovery, synthetic biology advancements |
| Manufacturing & Operations | Raw materials, utilities, facility upkeep, capital expenditure | Site expansions (e.g., UK Sandwich), capacity increases (peptide production) |
| Personnel Costs | Skilled workforce salaries, benefits, training | Attracting and retaining specialized scientific and technical talent |
| Quality & Regulatory Compliance | Testing, audits, certifications, documentation | Adherence to FDA, EMA, NMPA standards; GMP maintenance |
Revenue Streams
Asymchem's main income comes from charging clients for developing and manufacturing drug substances, like active pharmaceutical ingredients (APIs), peptides, and oligonucleotides. These fees are determined by how complicated the project is, how much product is needed, and which development phase they are supporting, from early research to full commercial production.
For instance, in 2024, Asymchem reported significant revenue growth from its CDMO services, a testament to the demand for its expertise in complex drug substance development and manufacturing. The company's ability to handle diverse projects, from small-scale clinical trial materials to large commercial batches, allows for flexible pricing structures that reflect the value delivered at each stage.
Asymchem generates significant revenue from offering comprehensive drug product development and manufacturing services. This includes crucial stages like formulation development, where they help clients create stable and effective drug delivery systems, and analytical method development, ensuring the quality and consistency of the final product. These integrated services go beyond simply producing the active pharmaceutical ingredient (API), providing a more complete solution for their clients.
By offering these end-to-end services, Asymchem captures additional value per project, as clients benefit from a streamlined process from early-stage development through to fill-finish manufacturing. This expansion into the drug product phase allows for deeper client relationships and a more substantial revenue contribution from each partnership, solidifying their position as a full-service CDMO.
Asymchem generates significant income through agreements to supply materials for both clinical trials and commercially available drugs. These typically long-term contracts offer a predictable and consistent revenue flow, particularly as more of their projects move into later development phases and reach the market.
For instance, in the first half of 2024, Asymchem reported a substantial increase in revenue from its CDMO services, driven by these very supply agreements. Their ability to secure and fulfill these contracts underpins a core component of their financial stability and growth strategy.
Revenue from Technology Transfer and Licensing
Asymchem's strong foundation in advanced manufacturing technologies and its significant patent portfolio indicate a potential for revenue generation through technology transfer and licensing. This could involve granting access to proprietary processes or specialized equipment to other companies for their specific manufacturing needs.
While not a publicly emphasized revenue stream, such agreements would leverage Asymchem's intellectual property and technical know-how. For example, in 2024, the company continued to invest heavily in research and development, further expanding its technological capabilities.
- Leveraging Proprietary Processes: Asymchem can license its unique chemical synthesis routes or manufacturing techniques to other pharmaceutical or biotech companies.
- Equipment Licensing: The company might license the use of specialized, proprietary manufacturing equipment it has developed or optimized.
- Collaborative Development: Licensing agreements could be structured as part of broader collaborations, sharing risks and rewards in developing new therapeutic modalities.
Service Fees for Analytical, Regulatory, and R&D Support
Asymchem generates additional revenue through specialized services beyond its core manufacturing. These include analytical testing to ensure product quality and compliance, and regulatory support crucial for clients navigating IND and NDA filings with agencies like the FDA.
Furthermore, the company offers targeted R&D consulting, leveraging its extensive scientific expertise to assist clients with specific development challenges. These value-added services capitalize on Asymchem's deep industry knowledge, providing essential support that complements its contract development and manufacturing services.
- Analytical Testing: Provides clients with critical data for quality control and regulatory submissions.
- Regulatory Support: Assists in the preparation and submission of Investigational New Drug (IND) and New Drug Application (NDA) filings.
- R&D Consulting: Offers specialized expertise for complex research and development projects.
- Value-Added Services: These ancillary offerings enhance client relationships and create diversified income streams.
Asymchem's revenue streams are primarily driven by its Contract Development and Manufacturing Organization (CDMO) services, encompassing the development and production of complex drug substances like APIs, peptides, and oligonucleotides. These services are priced based on project complexity, scale, and the specific development phase, from early research to commercial manufacturing.
In 2024, Asymchem saw robust growth in its CDMO business, reflecting strong demand for its expertise in handling intricate projects and diverse manufacturing scales. The company's ability to provide integrated services, including formulation development and analytical method development, allows them to capture additional value and foster deeper client relationships.
The company also generates consistent revenue from long-term supply agreements for both clinical trial materials and commercial drugs, providing a predictable income flow. Asymchem's investment in R&D in 2024 further bolsters its potential for revenue generation through technology transfer and licensing of proprietary processes and equipment.
Business Model Canvas Data Sources
The Asymchem Business Model Canvas is built upon a foundation of extensive market research, internal operational data, and financial performance metrics. These sources provide a comprehensive view of our current capabilities and future opportunities.
