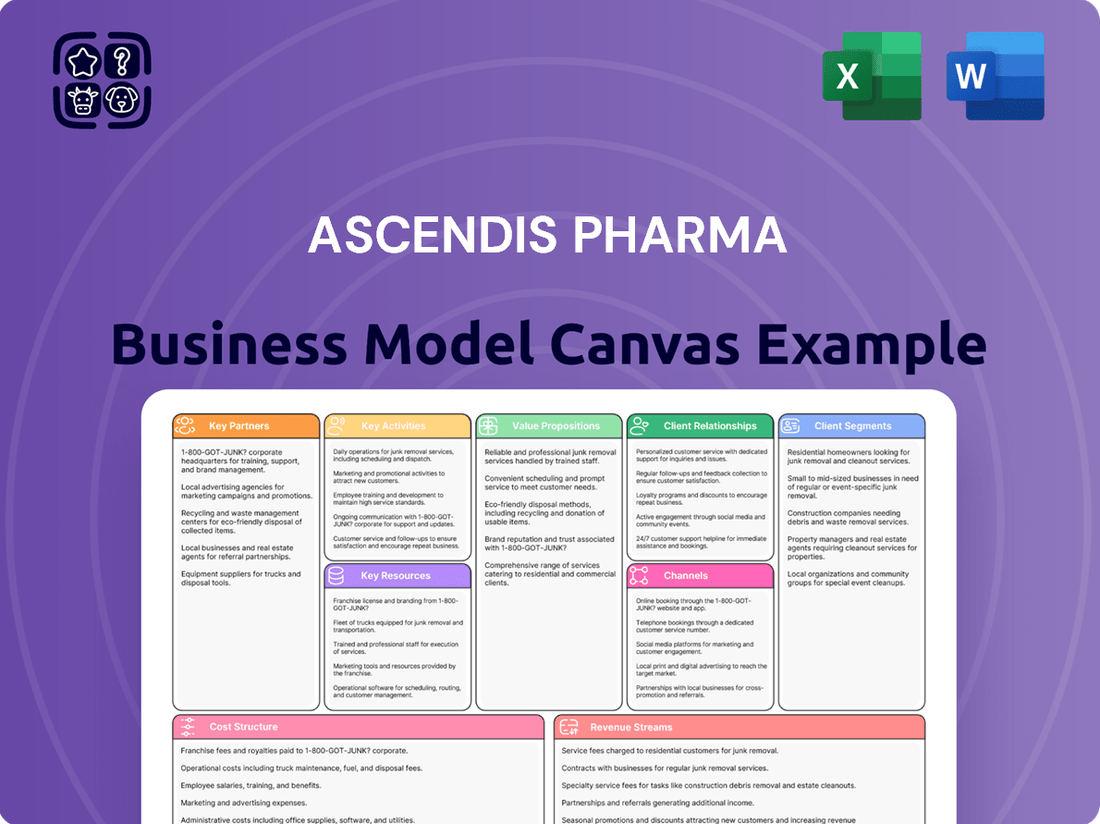
Ascendis Pharma Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Ascendis Pharma Bundle
Uncover the strategic engine behind Ascendis Pharma's success with our comprehensive Business Model Canvas. This detailed analysis breaks down their innovative approach to value creation, customer relationships, and revenue streams, offering a clear roadmap for industry players. Download the full canvas to gain actionable insights for your own strategic planning and competitive advantage.
Partnerships
Ascendis Pharma engages in strategic collaborations with other leading pharmaceutical firms, notably Novo Nordisk. These alliances are designed to harness Ascendis's innovative TransCon technology for a wider range of therapeutic uses, extending into metabolic and cardiovascular conditions. This partnership model helps distribute the significant costs associated with drug development and research, while also opening doors to new patient populations and markets.
Ascendis Pharma heavily utilizes Contract Manufacturing Organizations (CMOs) for its product and candidate production. This strategy allows for flexible scaling of manufacturing operations and avoids significant upfront investment in production facilities, a common practice in the pharma sector. For instance, in 2024, Ascendis continued to leverage CMOs for its key pipeline products, ensuring robust supply chains while maintaining high quality and regulatory compliance.
Ascendis Pharma's strategic collaborations with academic and research institutions are foundational for its innovation engine. These partnerships are crucial for the early-stage research and preclinical development of novel therapies, allowing Ascendis to explore new applications for its proprietary TransCon technology. For instance, in 2024, Ascendis continued to engage with leading universities and research centers globally to identify unmet medical needs and potential therapeutic targets, a strategy that has historically fueled its pipeline growth and scientific breakthroughs.
Clinical Research Organizations (CROs)
Ascendis Pharma relies on Clinical Research Organizations (CROs) to expertly manage and execute its clinical trials across all phases, from initial testing to large-scale efficacy studies.
These partnerships are crucial for leveraging specialized knowledge in areas like trial design, identifying and enrolling suitable patients, meticulously collecting data, and navigating complex regulatory submission processes. This collaboration significantly speeds up the timeline for bringing new therapies to market.
By outsourcing these critical operational aspects to CROs, Ascendis Pharma can concentrate its internal resources on its core strengths: innovative drug discovery and the overarching development strategy. This ensures that clinical studies are conducted with the highest standards of rigor and compliance.
For instance, in 2024, Ascendis Pharma continued to deepen its engagement with leading CROs to advance its pipeline, particularly in oncology and rare diseases. The efficiency gains from these partnerships are vital, as the average cost of a Phase 3 clinical trial can exceed $50 million, making optimized execution paramount.
- Expertise in Trial Execution: CROs provide specialized skills in patient recruitment, data management, and regulatory affairs.
- Accelerated Development: Partnerships with CROs help to streamline the clinical trial process, reducing time to market.
- Focus on Core Competencies: Outsourcing clinical trial management allows Ascendis to concentrate on drug discovery and development.
- Ensuring Compliance and Rigor: CROs ensure that all clinical studies adhere to strict regulatory standards and maintain scientific integrity.
Patient Advocacy Groups and Foundations
Ascendis Pharma actively partners with patient advocacy groups and foundations. These collaborations are vital for gaining deep insights into the unmet needs of patients and for increasing general awareness about specific diseases. For instance, in 2024, Ascendis Pharma continued its engagement with various rare disease foundations, leveraging their expertise to refine clinical trial designs and patient recruitment strategies.
These partnerships extend to supporting patient access programs, which are crucial for ensuring that individuals can obtain and afford necessary therapies. By working with these organizations, Ascendis Pharma can better understand and navigate the complexities of reimbursement and market access, ultimately benefiting the patient community. The company's commitment to these groups underscores a patient-centric approach to drug development and commercialization.
- Patient Needs Identification: Collaboration with groups like the National Organization for Rare Disorders (NORD) helps Ascendis Pharma pinpoint critical gaps in current treatment options and understand the daily challenges faced by patients.
- Disease Awareness Campaigns: In 2024, Ascendis Pharma supported initiatives aimed at raising public and physician awareness for conditions like hypoparathyroidism, often in conjunction with dedicated foundations.
- Patient Support Programs: These partnerships facilitate the development and delivery of crucial support services, including educational resources and financial assistance navigation, for patients undergoing treatment.
- Clinical Trial Engagement: Advocacy groups provide invaluable input on trial protocols and patient recruitment, ensuring that studies are designed with patient well-being and feasibility in mind.
Ascendis Pharma's key partnerships are crucial for its innovative business model, focusing on collaborations that accelerate drug development and broaden market reach. These alliances span strategic co-development with major pharmaceutical players, leveraging specialized contract manufacturing organizations (CMOs) for production, and deep engagement with academic institutions for early-stage research. Furthermore, partnerships with Clinical Research Organizations (CROs) are vital for efficient trial execution, while collaborations with patient advocacy groups ensure a patient-centric approach and facilitate market access.
| Partner Type | Purpose | Example/Impact |
| Pharmaceutical Firms (e.g., Novo Nordisk) | Co-development, market expansion | Leveraging TransCon technology for metabolic/cardiovascular conditions, sharing development costs. |
| Contract Manufacturing Organizations (CMOs) | Product manufacturing, flexible scaling | Ensuring robust supply chains for key pipeline products while maintaining quality and compliance in 2024. |
| Academic & Research Institutions | Early-stage research, preclinical development | Identifying unmet medical needs and potential therapeutic targets for new applications of TransCon technology. |
| Clinical Research Organizations (CROs) | Clinical trial management and execution | Accelerating development timelines; average Phase 3 trial cost can exceed $50 million, making optimized execution vital. |
| Patient Advocacy Groups | Patient needs insight, disease awareness, access programs | Refining clinical trial designs and patient recruitment strategies, supporting patient access in 2024. |
What is included in the product
This Ascendis Pharma Business Model Canvas provides a comprehensive, pre-written overview of their strategy, detailing customer segments, channels, and value propositions to reflect real-world operations.
It is organized into 9 classic BMC blocks with full narrative and insights, designed to help entrepreneurs and analysts make informed decisions and supports validation of business ideas using real company data.
Ascendis Pharma's Business Model Canvas acts as a pain point reliever by quickly identifying core components with a one-page business snapshot, streamlining complex drug development processes.
It provides a clean and concise layout ready for boardrooms or teams, saving hours of formatting and structuring for efficient strategic planning.
Activities
Ascendis Pharma's core activity revolves around intensive research and development, focusing on discovering and advancing innovative prodrugs through its proprietary TransCon technology. This commitment fuels their pipeline, encompassing preclinical studies, multi-phase clinical trials, and exploration of new therapeutic frontiers, particularly in oncology.
In 2024, Ascendis Pharma continued to invest heavily in R&D, a critical driver for biopharmaceutical growth. Their pipeline advancement, including ongoing trials for their oncology programs, underscores the strategic importance of this activity for future revenue generation and market positioning.
Ascendis Pharma's clinical development and regulatory submissions are a cornerstone of its business model. A primary activity involves meticulously managing and executing clinical trials for its innovative pipeline candidates, including TransCon CNP, TransCon hGH (marketed as SKYTROFA), and TransCon PTH (marketed as YORVIPATH). This encompasses the critical stages of patient recruitment, rigorous data analysis, and the preparation and submission of comprehensive regulatory applications, such as New Drug Applications (NDAs), supplemental Biologics License Applications (sBLAs), and Marketing Authorisation Applications (MAAs).
These submissions are directed to key global health authorities, notably the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The ultimate goal is to secure product approvals and achieve necessary label expansions, thereby unlocking the full commercial potential of their therapies. For instance, in 2023, Ascendis Pharma announced positive topline results from its Phase 3 trial for TransCon CNP in children with achondroplasia, a significant step towards regulatory submission.
Ascendis Pharma's manufacturing and supply chain management is centered on ensuring the efficient and compliant production of its innovative therapies. This includes overseeing the manufacturing of both commercial products and crucial clinical trial materials, a complex process requiring stringent quality control at every stage.
A significant aspect of this key activity involves managing relationships with third-party manufacturers. Ascendis Pharma relies on these partners to scale production, ensuring that its therapies can reach patients worldwide. For instance, in 2024, the company continued to build out its network of specialized contract manufacturing organizations to support its growing pipeline.
Establishing robust supply chain logistics is paramount to guaranteeing product availability. This encompasses everything from raw material sourcing to the final delivery of medicines to patients, navigating global regulatory requirements and ensuring timely access to life-changing treatments.
Commercialization and Market Access
Following regulatory approvals, Ascendis Pharma concentrates on the successful commercial launch and sustained marketing of its innovative therapies, exemplified by SKYTROFA and YORVIPATH. This critical phase involves establishing robust commercial infrastructure, fostering engagement with healthcare providers, and developing effective pricing and reimbursement strategies.
Ensuring broad patient access is paramount, which Ascendis achieves through meticulous management of distribution channels. For instance, in 2024, the company continued to expand the reach of its approved products, aiming to solidify market presence and drive revenue growth through targeted commercialization efforts.
- Commercial Launch: Executing go-to-market strategies for new therapies post-approval.
- Market Access: Securing favorable pricing and reimbursement from payers.
- Healthcare Provider Engagement: Educating physicians and key opinion leaders on product benefits.
- Distribution Management: Establishing efficient supply chains to ensure product availability.
Intellectual Property Management
Ascendis Pharma's intellectual property management is centered on safeguarding its groundbreaking TransCon technology and its pipeline of innovative therapies. This involves securing robust patent protection, trademarks, and other legal rights to maintain a distinct competitive edge.
This strategic IP protection is fundamental to maximizing the commercial value of Ascendis Pharma's therapeutic advancements. It lays the groundwork for sustained growth and opens avenues for lucrative licensing agreements and partnerships.
In 2024, Ascendis Pharma continued to actively manage its extensive patent portfolio. For instance, the company held over 1,000 granted patents globally by mid-2024, covering various aspects of its TransCon technology and drug candidates.
- Patent Protection: Ascendis Pharma focuses on obtaining and maintaining patents for its TransCon technology and specific drug candidates, ensuring exclusivity for its therapies.
- Trademark Registration: The company registers trademarks for its brand names and product identifiers to protect its market identity.
- Freedom-to-Operate Analysis: Conducting regular analyses to ensure its products do not infringe on existing third-party patents is a key activity.
- IP Enforcement: Actively monitoring and enforcing its intellectual property rights against potential infringements to preserve its market position.
Ascendis Pharma's key activities are deeply rooted in innovation and commercialization. The company prioritizes rigorous research and development to advance its TransCon technology-based pipeline, particularly in oncology. It also focuses on navigating the complex regulatory landscape through meticulous clinical trial management and timely submissions to global health authorities like the FDA and EMA. Furthermore, Ascendis manages its manufacturing and supply chain, often leveraging third-party partners, to ensure consistent product availability, and executes strategic commercial launches and marketing efforts for its approved therapies, such as SKYTROFA and YORVIPATH, to drive market penetration and revenue growth.
| Key Activity | Description | 2024 Focus/Data Points |
|---|---|---|
| Research & Development | Discovering and advancing prodrugs via TransCon technology. | Continued investment in pipeline advancement, especially oncology programs. |
| Clinical Development & Regulatory | Managing clinical trials and submitting regulatory applications. | Advancing trials for key candidates; aiming for regulatory submissions post-positive data. |
| Manufacturing & Supply Chain | Overseeing production and ensuring product availability. | Expanding network of third-party manufacturers for growing pipeline. |
| Commercial Launch & Marketing | Launching and marketing approved therapies. | Expanding reach of SKYTROFA and YORVIPATH, solidifying market presence. |
| Intellectual Property Management | Protecting TransCon technology and therapies via patents. | Managing over 1,000 granted patents globally by mid-2024. |
Full Version Awaits
Business Model Canvas
The Ascendis Pharma Business Model Canvas preview you are viewing is the exact document you will receive upon purchase. This means you're seeing the actual structure, content, and formatting that will be delivered, ensuring no surprises and full transparency. Once your order is complete, you'll gain immediate access to this comprehensive Business Model Canvas, ready for your strategic analysis and planning.
Resources
Ascendis Pharma's proprietary TransCon Technology Platform is the bedrock of its innovation, allowing for the creation of sustained-release prodrugs. This patented technology is crucial for developing therapies with enhanced drug profiles across various medical fields. In 2024, Ascendis continued to leverage this platform, with key pipeline advancements highlighting its ongoing strategic importance.
Ascendis Pharma's intellectual property, particularly its patents covering the TransCon technology and key product candidates like SKYTROFA and YORVIPATH, is a cornerstone of its business model. These patents grant crucial market exclusivity, safeguarding Ascendis Pharma's competitive advantage and investment in research and development.
The company's patent portfolio is extensive, with numerous granted patents and pending applications worldwide. For instance, as of early 2024, Ascendis Pharma held a significant number of patents related to its TransCon linker technology, which is fundamental to its product pipeline and ensures long-term protection for its innovative drug delivery system.
Licensing agreements, such as the strategic collaboration with Novo Nordisk for the development and commercialization of TransCon hGH, are also vital intellectual assets. These partnerships not only provide access to external expertise and market reach but also generate revenue streams, further solidifying the value of Ascendis Pharma's intellectual property.
Ascendis Pharma’s business model hinges on its highly specialized human capital. This includes R&D scientists, clinical development experts, regulatory affairs professionals, and dedicated commercial teams. Their collective expertise is crucial for navigating the complexities of drug discovery, clinical trials, and market entry.
The company’s success is directly tied to the deep knowledge of its workforce in areas like gene therapy and endocrinology. For instance, in 2024, Ascendis Pharma continued to invest heavily in its scientific talent, recognizing that innovation in these specialized fields requires top-tier researchers and developers.
Cash and Marketable Securities
Ascendis Pharma's cash and marketable securities are a critical resource, directly fueling its extensive research and development pipeline, crucial clinical trials, and the complex processes of manufacturing and commercialization. This financial bedrock is essential for bringing innovative therapies to market.
As of March 31, 2025, Ascendis Pharma maintained a robust financial position, reporting a substantial cash and cash equivalents balance. This healthy liquidity ensures the company can comfortably cover its operational expenses and pursue strategic growth opportunities without immediate funding constraints.
- Financial Foundation: Significant cash reserves provide the necessary capital for long-term R&D investments and operational continuity.
- Operational Flexibility: Ample liquidity allows Ascendis Pharma to navigate the inherent uncertainties of drug development and adapt to market dynamics.
- Strategic Investment: Cash on hand enables the company to pursue strategic acquisitions, partnerships, or internal development projects that align with its growth objectives.
Approved and Pipeline Products
Ascendis Pharma's key resources are anchored by its innovative approved and pipeline products. SKYTROFA, for example, is a long-acting growth hormone therapy, and YORVIPATH is a treatment for a rare genetic disorder. These represent the tangible results of significant research and development investment, forming the bedrock of their commercial efforts.
The company's pipeline is equally crucial, featuring promising candidates such as TransCon CNP, which targets achondroplasia. This robust pipeline indicates substantial future revenue potential and a commitment to addressing critical unmet medical needs across various therapeutic areas.
- Approved Products: SKYTROFA and YORVIPATH are generating revenue and establishing market presence.
- Pipeline Strength: TransCon CNP and other candidates in development offer significant future growth prospects.
- Addressing Unmet Needs: Ascendis Pharma's portfolio targets rare diseases and conditions with limited treatment options.
- R&D Output: These products are the direct outcome of the company's focused research and development strategy.
Ascendis Pharma's key resources are its groundbreaking TransCon Technology Platform, a robust intellectual property portfolio protecting its innovations, and its highly skilled workforce. These elements are critical for developing and commercializing its therapeutic products.
The company's financial stability, evidenced by significant cash reserves, underpins its ability to fund extensive research, clinical trials, and market expansion. Approved products like SKYTROFA and YORVIPATH, alongside a promising pipeline including TransCon CNP, represent the tangible output of these investments and are central to Ascendis Pharma's value proposition.
| Resource Category | Key Assets | 2024/2025 Significance |
|---|---|---|
| Technology Platform | TransCon Technology | Foundation for sustained-release prodrugs; ongoing pipeline advancements in 2024. |
| Intellectual Property | Patents on TransCon technology and products (SKYTROFA, YORVIPATH) | Market exclusivity, competitive advantage; extensive global patent coverage as of early 2024. |
| Human Capital | R&D scientists, clinical experts, regulatory professionals | Crucial for drug discovery, trials, and market entry; continued investment in talent in 2024. |
| Financial Resources | Cash and marketable securities | Fueling R&D, trials, manufacturing; substantial cash balance as of March 31, 2025. |
| Products & Pipeline | SKYTROFA, YORVIPATH, TransCon CNP | Revenue generation, market presence, future growth prospects addressing unmet medical needs. |
Value Propositions
Ascendis Pharma's TransCon technology is designed to significantly boost how well treatments work and improve what happens to patients. By releasing drugs steadily and fine-tuning how they behave in the body, Ascendis aims for better results, especially in areas like rare diseases and cancer where existing options might not be ideal.
This focus on improved treatment efficacy is a core part of their value proposition. For instance, their drug Skytrofa (lonapegesis-lysin) for growth hormone deficiency, approved in 2021, offers a once-weekly injection, a marked improvement over daily injections for many patients, demonstrating the practical benefits of their platform.
Ascendis Pharma's TransCon technology significantly boosts patient convenience and treatment adherence. For instance, SKYTROFA, a once-weekly treatment for growth hormone deficiency, dramatically cuts down on daily injections, a major hurdle for many patients with chronic conditions. This simplified regimen directly tackles adherence issues, a critical factor in successful long-term disease management.
Ascendis Pharma targets rare diseases, a segment often overlooked by larger pharmaceutical companies due to smaller patient populations. This focus allows them to address critical gaps in care where few or no effective treatments exist, directly impacting patient lives.
Their pipeline exemplifies this commitment, featuring promising therapies for conditions like achondroplasia and hypoparathyroidism. For achondroplasia, a genetic disorder affecting bone growth, Ascendis's drug candidate has shown significant efficacy in clinical trials, offering a novel therapeutic approach for affected individuals.
In 2024, the rare disease market continued its robust growth, projected to reach over $250 billion globally. Ascendis's strategic positioning within this expanding sector, particularly with its late-stage assets, underscores its potential to capture significant market share by meeting these urgent, unmet medical needs.
Differentiated Product Profiles
Ascendis Pharma's TransCon technology underpins its differentiated product profiles, offering tangible benefits over current treatments. These advantages often translate to enhanced safety, better patient tolerability, and optimized pharmacokinetic properties, crucial for market penetration.
This focus on superior product attributes allows Ascendis to carve out unique positions in crowded therapeutic areas. For instance, their lead product candidate, TransCon PTH, aims to provide a more convenient and potentially safer alternative for treating hypoparathyroidism compared to existing daily injections.
The strategy is designed to achieve best-in-class status, which is critical for capturing market share and commanding premium pricing. This differentiation is a core element of their business model, driving value and investor interest.
- TransCon Technology: Enables unique drug delivery and formulation for improved patient outcomes.
- Key Advantages: Enhanced safety, tolerability, and pharmacokinetic profiles compared to competitors.
- Market Positioning: Aims for best-in-class status in target therapeutic areas.
- Example: TransCon PTH offers a potential improvement over existing hypoparathyroidism treatments.
Potential for Combination Therapies
The TransCon platform's inherent flexibility enables the creation of innovative combination therapies. This approach is exemplified by Ascendis Pharma's development of TransCon CNP and TransCon hGH for achondroplasia. These dual-acting treatments aim to deliver synergistic benefits, potentially leading to superior patient outcomes compared to single-agent therapies.
This strategic focus on combination therapies unlocks significant potential for further pipeline expansion and enhanced therapeutic utility. By leveraging the TransCon technology, Ascendis Pharma can explore novel treatment paradigms across various indications.
- Synergistic Benefits: Combination therapies can offer enhanced efficacy and potentially reduce side effects by targeting multiple pathways.
- Pipeline Expansion: The platform supports the development of multiple drug candidates, creating a robust pipeline.
- Improved Patient Outcomes: Combining treatments may lead to more comprehensive disease management and better quality of life for patients.
- Innovation Pathway: This approach positions Ascendis Pharma at the forefront of therapeutic innovation, addressing unmet medical needs.
Ascendis Pharma's value proposition centers on its innovative TransCon technology, which enables sustained drug release and improved therapeutic profiles. This platform aims to deliver best-in-class treatments, enhancing efficacy, safety, and patient convenience, particularly in rare diseases and oncology. The company's strategic focus on addressing significant unmet medical needs, exemplified by its pipeline, positions it to capture value in expanding therapeutic markets.
The market for rare disease treatments is substantial and growing, with projections indicating it will exceed $250 billion globally by 2024. Ascendis Pharma is well-positioned within this sector, with late-stage assets like TransCon PTH for hypoparathyroidism and TransCon hGH for growth hormone deficiency. These therapies offer distinct advantages, such as reduced dosing frequency, which can significantly improve patient adherence and quality of life.
Ascendis Pharma's commitment to developing combination therapies, like those for achondroplasia, further strengthens its value proposition by offering synergistic benefits and novel treatment paradigms. This approach allows for more comprehensive disease management and creates opportunities for pipeline expansion, addressing complex medical challenges with innovative solutions.
| Therapeutic Area | Key Product Candidate | TransCon Technology Benefit | Market Opportunity (2024 Projection) |
|---|---|---|---|
| Growth Hormone Deficiency | Skytrofa (lonapegesis-lysin) | Once-weekly injection vs. daily | Significant market share in pediatric endocrinology |
| Hypoparathyroidism | TransCon PTH | Potential for improved convenience and safety over daily injections | Addressing a critical unmet need in endocrinology |
| Achondroplasia | TransCon hGH / TransCon CNP | Dual-acting combination therapy for improved bone growth | Targeting a rare genetic disorder with limited treatment options |
Customer Relationships
Ascendis Pharma cultivates direct connections with healthcare professionals, particularly endocrinologists and specialists in rare diseases, via its dedicated sales teams and Medical Science Liaisons. This direct engagement facilitates thorough education on product advantages, appropriate patient identification, and continuous support for effective patient care.
In 2024, Ascendis Pharma's sales force played a crucial role in educating physicians about their innovative therapies, contributing to market penetration and physician adoption. The company's commitment to building these relationships ensures that medical professionals are well-informed about the benefits and proper utilization of their treatments.
Ascendis Pharma's commitment to patient well-being is evident in its personalized support programs, like the Ascendis Signature Access Program (ASAP). These initiatives are designed to help patients overcome barriers to accessing vital therapies, focusing on navigating complex insurance and reimbursement landscapes.
The ASAP program exemplifies Ascendis's dedication to patient success, offering tailored assistance that goes beyond simply providing medication. This proactive approach aims to ensure that patients can benefit from their treatments without undue financial or administrative burdens.
Ascendis Pharma actively cultivates relationships with Key Opinion Leaders (KOLs) within its therapeutic areas to foster awareness and adoption of its innovative treatments. These engagements are vital for educating the broader medical community and influencing treatment protocols.
In 2024, Ascendis Pharma continued to leverage scientific advisory boards and participation in major medical conferences, such as the European Association for the Study of Diabetes (EASD) Annual Meeting, to connect with influential physicians and researchers. Such interactions are critical for validating clinical data and building trust.
The company's commitment to scientific exchange, including the publication of clinical trial results in peer-reviewed journals, further solidifies its relationships with KOLs. This transparent approach ensures that leading medical professionals are well-informed about Ascendis' pipeline and approved therapies.
Digital and Online Engagement
Ascendis Pharma actively engages patients, caregivers, and healthcare professionals through its digital and online presence. Their company website serves as a central hub, offering detailed information on diseases and the company's innovative therapies. This digital strategy is crucial for disseminating knowledge and fostering understanding within the patient and medical communities.
The company leverages various online resources to educate its audience. This includes providing accessible educational materials that explain complex medical conditions and treatment options. By making this information readily available online, Ascendis Pharma empowers individuals to make more informed decisions about their health.
- Digital Platforms: Company website, social media channels, and dedicated online portals.
- Educational Content: Disease awareness materials, therapy information, scientific publications, and webinars.
- Target Audiences: Patients, caregivers, healthcare professionals (HCPs), and researchers.
- Engagement Goals: Enhance disease understanding, promote therapy adoption, and build community support.
Strategic Partnerships with Payers and Reimbursement Bodies
Ascendis Pharma prioritizes strategic partnerships with payers and reimbursement bodies to guarantee access for its innovative therapies. This engagement is crucial for demonstrating the significant clinical and economic value of their treatments, thereby securing favorable reimbursement decisions for patients. For instance, in 2024, successful negotiations with key European health technology assessment (HTA) bodies were vital for the market access of their growth hormone deficiency therapy.
- Engaging National & Regional Payers: Ascendis Pharma actively collaborates with national and regional payers and reimbursement authorities to ensure broad coverage for its high-value therapies.
- Demonstrating Value: The company focuses on presenting robust clinical trial data and pharmacoeconomic analyses to prove the therapeutic and cost-effectiveness of its products.
- Securing Reimbursement: These efforts are directly aimed at obtaining positive reimbursement decisions, which are essential for patient access to Ascendis Pharma's treatments.
- 2024 Market Access Successes: In 2024, Ascendis Pharma reported successful reimbursement agreements in several key European markets, facilitating patient access to their innovative endocrinology treatments.
Ascendis Pharma fosters deep relationships with healthcare professionals through direct engagement via sales teams and Medical Science Liaisons, ensuring thorough education on their therapies. In 2024, this direct physician education was instrumental in driving market penetration and adoption of their innovative treatments.
Patient support is a cornerstone, exemplified by programs like the Ascendis Signature Access Program (ASAP), designed to navigate insurance complexities and reduce patient access barriers. This personalized assistance underscores Ascendis's commitment to ensuring patients can benefit from their treatments without undue financial or administrative hurdles.
The company actively cultivates relationships with Key Opinion Leaders (KOLs) and engages in scientific exchange, including publications and conference participation like the 2024 EASD meeting, to build trust and influence treatment protocols.
Ascendis Pharma also leverages digital platforms, including its website, to disseminate disease awareness and therapy information to patients, caregivers, and HCPs, promoting understanding and informed decision-making.
Channels
Ascendis Pharma relies on specialty pharmacies and distributors to get its complex therapies to patients. These partners are crucial because they have the specialized infrastructure needed for products like biologics, which often require strict temperature control and careful handling. In 2024, the global specialty pharmacy market was projected to reach over $300 billion, highlighting the importance of these channels.
These channels provide more than just logistics; they offer vital patient support services. This includes helping patients navigate insurance, manage side effects, and adhere to treatment regimens, which is essential for the success of Ascendis's innovative treatments. The demand for patient support services in specialty pharmacy is growing, with many patients reporting that these services significantly improve their treatment experience.
Ascendis Pharma utilizes a direct sales force to build strong relationships with healthcare providers, focusing on specialists in endocrinology, rare diseases, and oncology. This approach ensures in-depth product education and crucial clinical support.
In 2024, Ascendis continued to invest in its specialized sales teams, recognizing their vital role in market penetration for its innovative therapies. This direct engagement allows for immediate feedback and tailored support to physicians, fostering trust and driving adoption.
Ascendis Pharma leverages hospital and clinic networks as a crucial distribution channel, particularly for its specialized therapies targeting rare diseases. This direct approach ensures that treatments reach the specialized medical centers where patients are most likely to receive care, optimizing accessibility and timely intervention.
In 2024, the pharmaceutical industry saw a continued emphasis on targeted therapies, with companies like Ascendis Pharma focusing on rare disease areas where patient populations are concentrated in specific healthcare facilities. This strategy allows for efficient delivery and specialized patient support.
Early Access Programs (Named Patient Programs)
Ascendis Pharma strategically employs early access programs, often referred to as named patient programs, to bridge the gap for patients with critical medical needs in regions where formal reimbursement pathways are still being established. This approach allows eligible individuals to receive Ascendis' innovative therapies while the company works through the broader commercialization and market access processes.
These programs are crucial for demonstrating product value and gathering real-world evidence, which can be instrumental in future regulatory and reimbursement discussions. For instance, in 2024, Ascendis continued to leverage these pathways for its pipeline candidates, ensuring that patients facing life-threatening conditions were not denied access due to administrative delays.
- Named Patient Programs: Facilitate early access to investigational or unapproved medicines for patients with serious or life-threatening conditions.
- Unmet Medical Needs: Addresses situations where no satisfactory alternative treatment exists.
- Real-World Evidence: Generates data on drug efficacy and safety in a clinical setting, supporting market access efforts.
- Global Reach: Expands patient access across various international markets awaiting full commercial approval.
International Distribution Agreements
Ascendis Pharma's international distribution agreements are a cornerstone of its global strategy, enabling the company to bring its innovative therapies to patients worldwide. These exclusive partnerships are crucial for navigating diverse regulatory landscapes and market dynamics.
By collaborating with established local distributors, Ascendis leverages their existing infrastructure and market knowledge. This approach accelerates market penetration and ensures effective commercialization of its products, such as the recently approved Skytrofa (lonapegsomatropin-tcgd) in key European markets through such agreements.
- Global Reach: Ascendis secures exclusive distribution rights with partners across numerous countries, aiming for broad patient access to its treatments.
- Local Expertise: Partnerships tap into the deep understanding of local markets by distributors, facilitating successful product launches and ongoing sales.
- Commercialization Focus: These agreements are designed to optimize the commercialization process, from regulatory approval to patient delivery.
- Revenue Generation: Distribution agreements are a primary channel for generating revenue from international sales, contributing significantly to the company's financial performance.
Ascendis Pharma utilizes specialty pharmacies and distributors as key channels, recognizing their essential role in delivering complex therapies. These partners possess the necessary infrastructure for handling specialized biologics, often requiring strict temperature controls. The global specialty pharmacy market was projected to exceed $300 billion in 2024, underscoring the significance of these distribution networks.
These channels also provide critical patient support services, assisting with insurance navigation and treatment adherence, which is vital for the success of Ascendis's innovative treatments. The demand for such support within specialty pharmacy continues to grow, with many patients reporting improved treatment experiences due to these services.
Ascendis Pharma's channel strategy also includes direct engagement with healthcare providers through a specialized sales force. This direct approach fosters strong relationships with endocrinologists, rare disease specialists, and oncologists, ensuring in-depth product education and clinical support.
In 2024, Ascendis continued to invest in its specialized sales teams to drive market penetration for its innovative therapies. This direct interaction facilitates immediate feedback from physicians and tailored support, building trust and encouraging product adoption.
Hospital and clinic networks serve as another crucial distribution channel for Ascendis, particularly for therapies targeting rare diseases. This strategy ensures treatments reach specialized medical centers where patients are most likely to receive care, optimizing accessibility and timely intervention.
The pharmaceutical industry in 2024 continued its focus on targeted therapies, with companies like Ascendis Pharma concentrating on rare disease areas where patient populations are often concentrated in specific healthcare facilities, enabling efficient delivery and specialized support.
Ascendis Pharma strategically employs early access programs, also known as named patient programs, to provide access to its therapies for patients with critical needs in regions awaiting formal reimbursement. These programs are instrumental in demonstrating product value and gathering real-world evidence to support future market access efforts.
In 2024, Ascendis continued to leverage these pathways for its pipeline candidates, ensuring that patients facing life-threatening conditions received access despite administrative delays.
International distribution agreements are fundamental to Ascendis Pharma's global strategy, enabling worldwide patient access to its innovative therapies. These exclusive partnerships are vital for navigating diverse regulatory environments and market complexities.
By partnering with established local distributors, Ascendis leverages their existing infrastructure and market insights to accelerate market penetration and ensure effective commercialization. This strategy was evident in the successful launch of Skytrofa in key European markets through such agreements.
| Channel Type | Key Function | 2024 Market Context/Example |
| Specialty Pharmacies & Distributors | Logistics, temperature control, patient support | Global specialty pharmacy market projected over $300 billion in 2024. |
| Direct Sales Force | Healthcare provider education, clinical support, relationship building | Continued investment in specialized teams for market penetration. |
| Hospital & Clinic Networks | Direct access for specialized therapies, patient care concentration | Focus on rare disease areas with concentrated patient populations in specific facilities. |
| Early Access Programs (Named Patient) | Bridging reimbursement gaps, real-world evidence generation | Used for pipeline candidates in 2024 to ensure patient access. |
| International Distribution Agreements | Global market access, leveraging local expertise, commercialization | Facilitated launches like Skytrofa in European markets. |
Customer Segments
Patients with rare endocrine diseases represent a core customer segment for Ascendis Pharma. This group includes individuals, both children and adults, diagnosed with Growth Hormone Deficiency (GHD). For these patients, SKYTROFA is a key therapeutic option, offering a long-term solution for their chronic condition.
Another significant portion of this segment comprises adults suffering from hypoparathyroidism. Ascendis Pharma addresses this need with YORVIPATH. These patients, much like those with GHD, face chronic health challenges necessitating ongoing medical management and treatment.
The market for rare endocrine diseases is substantial, with GHD affecting an estimated 1 in 3,000 to 1 in 4,000 live births. Hypoparathyroidism, while less common, impacts a notable number of adults, often following thyroid surgery or due to autoimmune causes.
Patients with achondroplasia, particularly children, represent a crucial customer segment for Ascendis Pharma. The company's TransCon CNP therapy is designed to address this significant unmet medical need in skeletal growth disorders, offering potential benefits as a standalone treatment or in conjunction with TransCon hGH.
Ascendis Pharma is actively developing innovative treatments for oncology patients, particularly those facing advanced or resistant forms of cancer. Their pipeline includes promising therapies like TransCon IL-2 β/γ, which is being investigated for conditions such as platinum-resistant ovarian cancer and melanoma, addressing significant unmet medical needs within these patient populations.
Healthcare Professionals (Physicians, Specialists)
Healthcare professionals, including endocrinologists, pediatric endocrinologists, rare disease specialists, and oncologists, form a core customer segment for Ascendis Pharma. These medical experts are the primary prescribers and managers of Ascendis' innovative therapies, making their adoption and understanding of the treatments critical for commercial success.
Their role is pivotal in the patient treatment journey. For instance, in 2024, the demand for specialized endocrinology services continued to grow, with an estimated 1.2 million new cases of diabetes diagnosed in the US alone, highlighting the need for advanced therapeutic options that Ascendis aims to provide.
- Key Prescribers: Physicians who directly influence patient treatment decisions.
- Specialized Focus: Endocrinologists and rare disease specialists are targeted due to Ascendis' pipeline.
- Treatment Pathway Influencers: Their expertise dictates the use of Ascendis' products.
Payers and Health Systems
Payers and health systems, encompassing government agencies like Medicare and Medicaid, alongside private insurers and managed care organizations, are pivotal decision-makers for Ascendis Pharma's products. Their coverage and reimbursement policies directly dictate patient access and the company's revenue streams. For instance, in 2024, the average list price for a new specialty drug could range from $200,000 to $300,000 annually, making payer negotiations paramount.
Securing favorable reimbursement is not merely a transactional process but a strategic imperative. Ascendis Pharma must demonstrate the clinical and economic value of its therapies to these entities. In 2023, the Inflation Reduction Act introduced measures impacting drug pricing negotiations for Medicare, highlighting the evolving landscape that payers navigate and influence.
- Payer Landscape: Includes government (Medicare, Medicaid), private insurers, and managed care organizations.
- Reimbursement Influence: Crucial for patient access and revenue generation for Ascendis Pharma's innovative therapies.
- Value Demonstration: Essential to prove clinical and economic benefits to secure favorable coverage decisions.
- Market Dynamics: Navigating evolving regulations, such as those introduced by the Inflation Reduction Act, impacts negotiation strategies.
Ascendis Pharma's customer base extends to patient advocacy groups and foundations that support rare disease communities. These organizations play a vital role in raising awareness, providing patient support, and influencing policy, all of which indirectly benefit Ascendis by fostering a favorable environment for their therapies.
Additionally, Ascendis Pharma engages with academic institutions and research organizations. Collaborations with these entities can lead to further clinical research, drug development, and a deeper understanding of the diseases they target, ultimately benefiting both the scientific community and the patients.
The company also targets potential partners for co-development or commercialization agreements. These strategic alliances can expand market reach and accelerate the availability of their treatments to a broader patient population.
Ascendis Pharma's customer segments can be broadly categorized as follows:
| Customer Segment | Description | Key Engagement Focus |
|---|---|---|
| Patients | Individuals with rare endocrine diseases (GHD, hypoparathyroidism) and skeletal growth disorders (achondroplasia), as well as oncology patients. | Providing innovative, long-term therapeutic solutions. |
| Healthcare Professionals | Endocrinologists, pediatric endocrinologists, rare disease specialists, and oncologists. | Educating on product efficacy, safety, and patient management. |
| Payers & Health Systems | Government agencies (Medicare, Medicaid), private insurers, managed care organizations. | Demonstrating clinical and economic value for reimbursement and market access. |
| Partners & Researchers | Patient advocacy groups, foundations, academic institutions, research organizations. | Fostering collaboration, awareness, and research advancements. |
Cost Structure
Research and Development (R&D) is a major cost driver for Ascendis Pharma, reflecting the significant investment required for drug discovery and clinical trials. These expenses are crucial for advancing their innovative therapies through the development pipeline.
In the first quarter of 2025, Ascendis Pharma reported R&D expenses totaling €86.6 million. This figure underscores the substantial financial commitment involved in preclinical research, extensive clinical testing, and the ongoing pursuit of novel drug candidates.
Selling, General, and Administrative (SG&A) expenses are crucial for Ascendis Pharma's business model, encompassing costs tied to bringing their therapies to market. This includes everything from marketing campaigns and sales force operations to essential patient support services and the general administrative functions that keep the company running smoothly.
The company's commitment to global commercial expansion has naturally led to an increase in these SG&A costs. For instance, in the first quarter of 2025, Ascendis Pharma reported SG&A expenses of €101 million, reflecting the significant investment in building out their commercial infrastructure worldwide.
Ascendis Pharma's manufacturing and supply chain costs are substantial, encompassing the production of active pharmaceutical ingredients (APIs) and the final drug product. These expenses also include rigorous quality control measures, specialized packaging, and the complex logistics of distributing their innovative therapies.
Given Ascendis Pharma's strategy of leveraging third-party manufacturers for a significant portion of its production, these outsourced costs represent a major expenditure. This approach allows for flexibility but necessitates careful management of contract manufacturing organizations (CMOs) to ensure quality and cost-effectiveness.
For instance, in 2024, the pharmaceutical industry's contract manufacturing market continued to grow, with many companies like Ascendis relying on these partners for API and drug product development and manufacturing. The specific figures for Ascendis's 2024 supply chain costs would be detailed in their annual financial reports, reflecting the significant investment in bringing their pipeline products to market.
Regulatory and Compliance Costs
Ascendis Pharma's cost structure includes significant expenses for regulatory and compliance activities. These are crucial for obtaining and maintaining approvals from health authorities worldwide, ensuring products can reach patients and remain on the market. For instance, preparing and submitting complex regulatory applications, such as New Drug Applications (NDAs) or Marketing Authorization Applications (MAAs), involves substantial investment in data generation, documentation, and expert consultation. In 2024, the pharmaceutical industry continued to see rising costs associated with navigating diverse and evolving regulatory landscapes. Post-market surveillance, including pharmacovigilance and ongoing safety monitoring, also represents a continuous financial commitment.
- Regulatory Application Fees: Costs associated with filing applications with agencies like the FDA, EMA, and others.
- Compliance Maintenance: Ongoing expenses for adhering to Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and other regulatory standards.
- Post-Market Surveillance: Investment in pharmacovigilance systems and reporting to monitor product safety after launch.
- Legal and Consulting Fees: Expenses for specialized legal and regulatory expertise to ensure compliance.
Intellectual Property and Legal Expenses
Ascendis Pharma incurs significant costs for patent prosecution and maintaining its intellectual property portfolio. These expenses are crucial for safeguarding their innovative drug candidates and proprietary technologies in the fiercely competitive biopharmaceutical landscape.
The company also allocates resources to potential litigation, a necessary measure to defend its intellectual property rights against infringement. This proactive legal stance protects their valuable assets and market exclusivity.
- Patent Prosecution: Costs associated with filing, prosecuting, and obtaining patents globally.
- Intellectual Property Maintenance: Ongoing fees to keep patents in force.
- Legal Defense: Expenses related to defending patents in case of challenges or litigation.
For instance, in 2023, Ascendis Pharma reported research and development expenses of €475.2 million, a significant portion of which is directly attributable to the development and protection of its intellectual property.
Ascendis Pharma's cost structure is heavily influenced by its substantial investments in research and development, essential for advancing its innovative therapies. Selling, General, and Administrative (SG&A) expenses are also significant, reflecting the costs associated with commercializing its products globally.
Manufacturing and supply chain costs, including those from contract manufacturers, represent another major expenditure. Furthermore, the company incurs considerable costs for regulatory compliance, patent prosecution, and intellectual property defense to protect its valuable assets.
| Cost Category | Q1 2025 (Millions €) | 2023 (Millions €) |
|---|---|---|
| Research & Development | 86.6 | 475.2 |
| Selling, General & Administrative | 101.0 | N/A |
| Manufacturing & Supply Chain | N/A | N/A |
| Regulatory & Compliance | N/A | N/A |
| Intellectual Property | N/A | N/A |
Revenue Streams
Ascendis Pharma's primary revenue stream originates from the direct sale of its approved commercial products. This includes key therapies like SKYTROFA, used for both pediatric and adult growth hormone deficiency, and YORVIPATH, which treats hypoparathyroidism. These product sales are a substantial driver of the company's financial performance.
In the first quarter of 2025, the impact of these sales was evident, with SKYTROFA contributing €51.3 million to revenue. YORVIPATH also demonstrated strong performance, generating €44.7 million during the same period. These figures highlight the commercial success and market adoption of Ascendis Pharma's innovative treatments.
Ascendis Pharma generates significant revenue through licensing its innovative TransCon technology. This involves upfront payments from partner companies, plus milestone payments tied to successful development and commercialization of partnered products.
A prime example is the $100 million upfront payment Ascendis received from Novo Nordisk for the global rights to Ascendis Pharma’s investigational drug for growth hormone deficiency. This highlights the substantial financial value attributed to their proprietary technology by major pharmaceutical players.
Ascendis Pharma also generates revenue from rendering services and clinical supply. This segment, while smaller than product sales, contributes to revenue diversification. For instance, in 2023, Ascendis Pharma reported total revenue of €566 million, with a portion attributed to these service-based activities and the supply of clinical materials to its partners.
Potential Future Product Launches
Ascendis Pharma anticipates significant revenue expansion from its pipeline, particularly with TransCon CNP for achondroplasia, which is currently under regulatory review. Successful market entry for this therapy is projected to be a key driver of future financial performance.
The company’s strategy hinges on the successful approval and commercialization of its late-stage assets. These upcoming product launches are expected to contribute substantially to revenue growth in the years following their market introduction.
- TransCon CNP for achondroplasia: Regulatory review ongoing, potential for significant revenue upon approval.
- Pipeline asset commercialization: Expected to drive substantial revenue growth in coming years.
- Future product launches: Key to expanding revenue streams and market presence.
Equity Investments/Holdings in Affiliates
Ascendis Pharma recognizes revenue and value from its equity stakes in affiliated companies. A prime example is its holding in VISEN Pharmaceuticals, which successfully completed an initial public offering (IPO) on the Hong Kong Stock Exchange. This strategic move allows Ascendis Pharma to potentially realize significant gains from its investment.
The value of these equity holdings can fluctuate based on market performance and the individual success of the affiliated companies. For instance, in 2023, Ascendis Pharma reported a gain on its investment in VISEN Pharmaceuticals, demonstrating the tangible financial benefit derived from such equity positions. This stream represents a crucial non-operating income source that complements its core business operations.
- Equity Investments in Affiliates: Revenue and value recognition from stakes in companies like VISEN Pharmaceuticals.
- VISEN Pharmaceuticals IPO: The public listing on the Hong Kong Stock Exchange in 2023 provided a significant valuation event for Ascendis Pharma's holding.
- Non-Operating Income Stream: These investments contribute to overall financial performance beyond core drug development and commercialization.
- Potential for Capital Gains: Ascendis Pharma can realize profits through the appreciation of its equity positions over time.
Ascendis Pharma's revenue is primarily driven by the sales of its approved therapies, SKYTROFA and YORVIPATH. These products address significant unmet medical needs, contributing substantially to the company's financial results. For example, in the first quarter of 2025, SKYTROFA generated €51.3 million and YORVIPATH brought in €44.7 million.
Beyond direct product sales, Ascendis Pharma leverages its innovative TransCon technology through licensing agreements. These partnerships provide upfront payments and future milestone revenues, as seen with the $100 million upfront payment from Novo Nordisk for a growth hormone deficiency drug candidate.
The company also diversifies its revenue through services and clinical supply to partners, contributing to overall financial stability. Furthermore, Ascendis Pharma recognizes value from its equity investments in affiliated companies, such as its stake in VISEN Pharmaceuticals, which went public in 2023.
| Revenue Stream | Description | Q1 2025 Contribution (Millions) | Key Examples |
| Product Sales | Direct sales of approved commercial products. | SKYTROFA: €51.3 YORVIPATH: €44.7 |
SKYTROFA, YORVIPATH |
| Licensing & Milestones | Revenue from licensing TransCon technology and achieving development milestones. | Upfront payments, milestone payments | Novo Nordisk partnership ($100M upfront) |
| Services & Clinical Supply | Revenue from providing services and clinical materials to partners. | Contributory | Partner collaborations |
| Equity Investments | Gains and value from stakes in affiliated companies. | Potential capital gains | VISEN Pharmaceuticals |
Business Model Canvas Data Sources
The Ascendis Pharma Business Model Canvas is informed by a robust combination of clinical trial data, regulatory filings, and market intelligence. This ensures a strategic foundation built on scientific progress and market opportunity.
