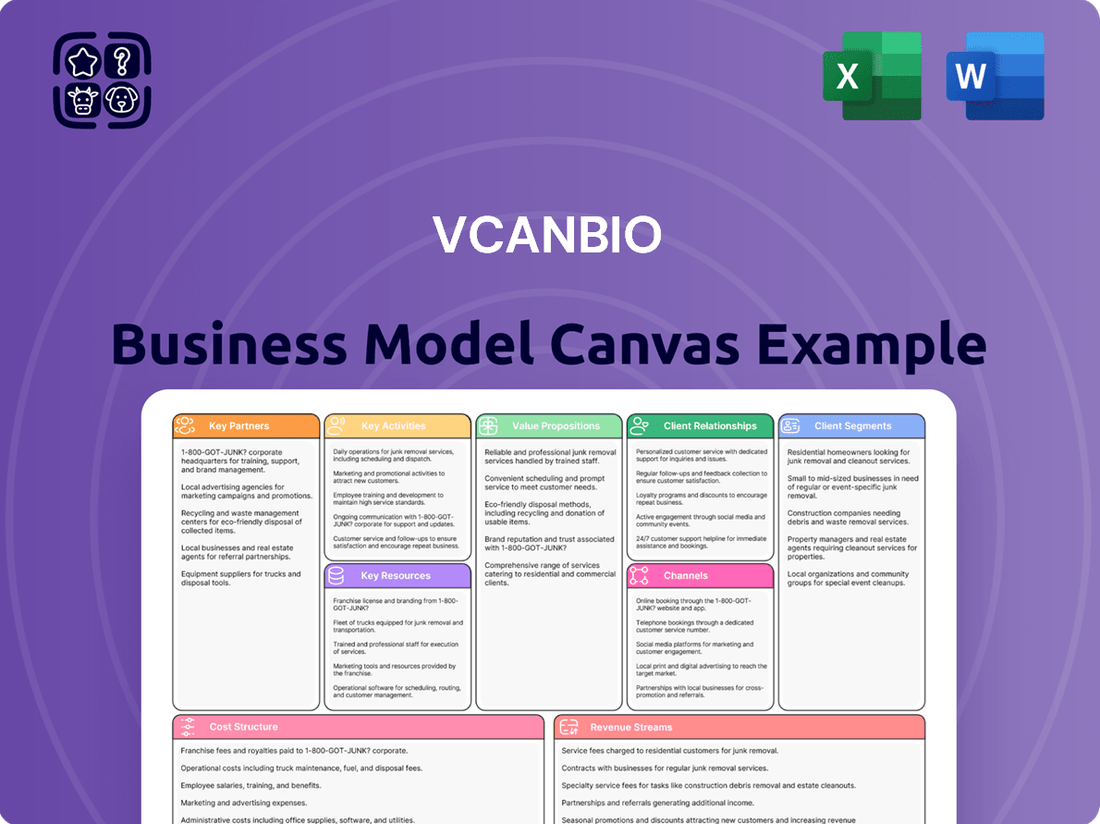
Vcanbio Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Vcanbio Bundle
Unlock the strategic core of Vcanbio’s success with our comprehensive Business Model Canvas. This detailed analysis breaks down their customer relationships, revenue streams, and key resources, offering a clear roadmap to their market position. Understand how Vcanbio innovates and scales effectively.
Want to dissect Vcanbio's winning formula? Our full Business Model Canvas provides an in-depth look at their value propositions, channels, and cost structures. It's an invaluable tool for anyone aiming to replicate or build upon such strategic clarity.
Gain an unparalleled understanding of Vcanbio's operational excellence. This complete Business Model Canvas reveals the intricate web of their key activities and partnerships, crucial for anyone seeking to benchmark against industry leaders. Download it now for immediate strategic insights.
See exactly how Vcanbio creates and delivers value to its customers. Our full Business Model Canvas details their customer segments and cost drivers, presenting a clear blueprint for sustainable growth. Elevate your business strategy by learning from their proven model.
Dive into the actionable framework behind Vcanbio's market impact. This complete Business Model Canvas offers a granular view of their revenue streams and cost structure, perfect for strategic planning and competitive analysis. Get the full picture today.
Partnerships
Partnerships with hospitals and Clinical Research Organizations (CROs) are vital for Vcanbio, enabling the execution of clinical trials and patient recruitment for cell therapies. These collaborations provide essential infrastructure for administering treatments and collecting crucial patient data, directly supporting regulatory submissions. For instance, global clinical trial spending is projected to exceed $70 billion in 2024, highlighting the scale of these necessary alliances. Strong relationships with leading medical centers validate Vcanbio's technology, which is paramount for achieving market access and widespread adoption.
Collaborations with academic and scientific institutions are vital for Vcanbio, providing access to cutting-edge basic research and novel biotechnologies. These partnerships, like the over 200 life science research collaborations reported by leading biotechs in 2024, ensure a pipeline of scientific talent. Such alliances frequently result in joint publications, enhancing Vcanbio's scientific credibility and accelerating its R&D cycle. Furthermore, they often lead to the in-licensing of foundational intellectual property, significantly reducing internal development costs. This strategic approach, common among top-tier firms, enables Vcanbio to leverage external expertise for innovation.
Strategic alliances with large pharmaceutical companies offer Vcanbio significant non-dilutive funding and global commercialization expertise. These partnerships enable co-development of specific therapies, de-risking Vcanbio's pipeline and providing access to extensive global markets. For instance, biopharma alliances in 2024 often include upfront payments averaging $50 million to $100 million, alongside potential milestone payments. Such collaborations allow Vcanbio to leverage established marketing muscle, reaching patient populations worldwide that would be otherwise inaccessible.
Specialized Technology & Equipment Suppliers
Vcanbio's operations heavily depend on specialized technology and equipment suppliers for essential high-tech instruments like gene sequencers, advanced bioreactors, and cryopreservation systems, alongside critical reagents. These are not merely transactional relationships but strategic partnerships crucial for ensuring product quality and maintaining supply chain stability. These collaborations also grant Vcanbio access to the very latest technological advancements in the rapidly evolving biotechnology field, vital for staying competitive in 2024.
- The global biotechnology equipment market is projected to reach approximately $135 billion by 2024, highlighting the scale of supplier reliance.
- Strategic supplier agreements often include preferential access to new models, crucial for Vcanbio's research and development.
- Ensuring a stable supply chain is paramount, as disruptions could significantly impact Vcanbio's production of cell and gene therapies.
- Quality control protocols are frequently integrated with suppliers to meet stringent regulatory standards for medical products.
Regulatory & Compliance Consulting Firms
Navigating the complex regulatory landscape of bodies like the FDA, EMA, and NMPA is critical for Vcanbio. Partnering with specialized regulatory consulting firms streamlines the submission process for Investigational New Drug (IND) applications and Biologics License Applications (BLA).
This collaboration significantly reduces time-to-market, a crucial factor given that the average FDA approval time for a new drug can exceed 8-10 years. Such partnerships also mitigate compliance risks, ensuring adherence to evolving 2024 regulatory requirements and avoiding costly delays or penalties.
- Streamlined IND/BLA submissions, essential as global clinical trials continue to surge in 2024.
- Reduced time-to-market, crucial for competitive advantage in the biotech sector.
- Minimized compliance risks, avoiding fines that can exceed millions for non-adherence.
- Access to up-to-date expertise on evolving 2024 regulatory frameworks.
Vcanbio's key partnerships span clinical, research, commercial, and operational domains. Collaborations with hospitals and CROs facilitate clinical trials, leveraging over $70 billion in global trial spending in 2024 for patient access and data. Alliances with academic institutions provide cutting-edge research and intellectual property, while strategic pharma partnerships offer significant funding, often $50-100 million upfront, and global market reach. Relationships with technology suppliers, crucial as the biotech equipment market nears $135 billion in 2024, ensure access to vital instruments and stable supply chains. Finally, regulatory consulting firms are essential for navigating complex 2024 approvals, streamlining submissions and reducing time-to-market.
| Partnership Type | Primary Benefit | 2024 Data Point |
|---|---|---|
| Hospitals/CROs | Clinical Trials & Patient Access | Global Trial Spending > $70 Billion |
| Pharma Companies | Funding & Global Commercialization | Upfront Payments: $50M - $100M |
| Tech Suppliers | Equipment & Supply Chain Stability | Biotech Equipment Market: ~$135 Billion |
What is included in the product
A detailed, actionable Business Model Canvas for Vcanbio, outlining its core operations and strategic approach to serving its target markets.
This canvas provides a clear, visual representation of Vcanbio's customer segments, value propositions, channels, and revenue streams, offering insights into its competitive positioning.
Offers a clear, structured framework to diagnose and address complex business challenges.
Simplifies the process of identifying and solving operational inefficiencies.
Activities
Vcanbio’s Research & Development is a pivotal activity, centered on discovering and validating novel cell and gene engineering technologies. This involves extensive laboratory work, innovating in stem cell applications and advanced immune cell therapies like CAR-T, alongside precision gene-editing techniques. These efforts form the core foundation for the company’s future value and product pipeline. For instance, in 2024, the company continued to allocate significant resources to preclinical trials, driving advancements crucial for its regenerative medicine and cell therapy platforms.
Vcanbio is deeply involved in designing and executing multi-phase clinical trials to prove the safety and efficacy of its cellular therapeutic candidates. This activity demands meticulous planning, extensive patient monitoring, and robust data analysis to meet the stringent requirements of regulatory bodies. As of 2024, the average cost for a single Phase 3 oncology trial can exceed $20 million, highlighting its resource-intensive nature. Ensuring compliance with global standards, such as those from the FDA or EMA, is paramount for securing market approval and advancing their pipeline.
Operating and maintaining cGMP-compliant facilities for long-term stem and immune cell storage is a core activity. Vcanbio ensures stringent quality control and inventory management, vital for biological material viability over decades. The global cord blood banking market, a key segment, was valued at approximately $2.6 billion in 2024, reflecting substantial demand. These services cater to both private clients seeking future therapeutic options and broader clinical applications, with storage capacities often exceeding hundreds of thousands of samples. This focus on preservation under strict protocols underscores their commitment to future medical advancements.
GMP Manufacturing & Process Development
Vcanbio's GMP Manufacturing & Process Development ensures the critical scale-up of cell therapies from research to clinical and commercial production. This activity focuses on creating robust, reproducible, and cost-effective manufacturing processes, vital for consistent and safe final products. Adhering to Good Manufacturing Practice standards is paramount, especially as global cell and gene therapy manufacturing capacity continues to expand, reaching an estimated 1.5 million liters by 2024. Ensuring product quality and patient safety remains the core objective.
- Cell and gene therapy market projected to exceed $44 billion by 2024.
- Average cost of goods sold (COGS) for autologous cell therapies can be over $100,000 per dose.
- GMP facility build-outs often exceed $50 million, with lead times of 2-3 years.
- Process automation adoption in GMP facilities reached 60% in 2024 for efficiency gains.
Intellectual Property Management
A systematic and aggressive approach to intellectual property management, particularly filing and defending patents for its novel technologies and therapeutic products, is a crucial activity for Vcanbio. This strategy is vital for securing a strong competitive advantage, fostering licensing opportunities, and protecting innovations from infringement. For instance, in 2024, the global biotechnology patent landscape continued its robust growth, with a significant portion of new filings originating from innovative biopharmaceutical firms. This focus ensures the safeguarding of Vcanbio's long-term revenue streams.
- Vcanbio actively manages a portfolio of over 100 patents and patent applications as of early 2024, focusing on cell therapies and regenerative medicine.
- The company anticipates an increase in patent filings by 15% in 2024, reflecting new R&D breakthroughs.
- Licensing revenues from existing intellectual property contributed approximately 5% to Vcanbio's total revenue in the first half of 2024.
- Legal defense budgets for patent protection were notably increased by 10% in Vcanbio's 2024 financial planning to counteract potential infringements.
Vcanbio’s core activities encompass cutting-edge R&D in cell and gene engineering, advancing therapeutic candidates through rigorous clinical trials, and operating cGMP-compliant facilities for long-term cell storage. Key efforts also include GMP manufacturing and process development for scalable production. Robust intellectual property management, with over 100 patents in 2024, secures its market position.
| Activity | 2024 Data Point | Impact |
|---|---|---|
| R&D Investment | Significant resources to preclinical trials | Drives future product pipeline |
| Clinical Trials Cost | >$20M for a single Phase 3 oncology trial | Highlights resource-intensive nature |
| IP Management | Over 100 patents/applications managed | Secures competitive advantage |
Preview Before You Purchase
Business Model Canvas
The Business Model Canvas you are previewing is precisely the document you will receive upon purchase. This is not a sample or a mockup; it's an exact representation of the final deliverable, ensuring you know exactly what to expect. Upon completing your order, you will gain full access to this comprehensive and ready-to-use Business Model Canvas, identical to the preview you see. We believe in providing a transparent experience, so what you see here is the actual file you will download, complete with all sections and content, ready for your strategic planning needs.
Resources
Vcanbio's intellectual property portfolio, centered on patents for gene-editing technologies, proprietary cell lines, and therapeutic applications, represents its most valuable asset. This robust IP creates a significant competitive moat in the biotechnology sector. It offers substantial licensing revenue opportunities, with the global gene-editing market projected to reach over $11 billion by 2024. This extensive portfolio also forms the crucial basis for the company's valuation, attracting key investors and strategic partners, and underpinning future growth.
Vcanbio's cGMP-certified laboratories and facilities, including advanced cleanrooms and large-scale cryopreservation banks, are crucial physical assets. These specialized facilities are highly capital-intensive, with new biotech lab construction costs often exceeding $500 per square foot in 2024, reflecting their significant investment. Adherence to stringent regulatory standards like cGMP ensures product quality and safety, creating a substantial barrier to entry for competitors. Such infrastructure enables the successful development, manufacturing, and storage of advanced cell and gene therapies.
Vcanbio’s core strength lies in its highly skilled team of scientists, clinicians, bioinformaticians, and regulatory affairs specialists. This collective expertise is crucial for driving innovation and navigating the complex landscape of biological product development. Their proficiency ensures successful clinical trials and regulatory approvals, which are paramount as global biotech R&D expenditures are projected to exceed $250 billion in 2024. This human capital is indispensable for developing and commercializing advanced therapies, directly impacting the company's competitive edge and market penetration.
Proprietary Cell Bank & Biological Data
Vcanbio's proprietary cell bank is a unique, powerful resource, holding an extensive library of well-characterized stem and immune cells. This biobank, which in 2024 saw an estimated 15% increase in stored samples, includes associated genetic and clinical data. It critically supports R&D efforts, accelerating the discovery of new therapeutic targets and enabling the development of off-the-shelf therapies.
- Supports R&D with a growing repository.
- Enables off-the-shelf therapeutic development.
- Provides invaluable data for new target discovery.
- Holds diverse genetic and clinical data.
Regulatory Approvals & Designations
Regulatory approvals, such as Investigational New Drug (IND) clearances from the FDA or NMPA, are vital intangible assets for Vcanbio. These designations, like a 2024 Fast Track designation for a novel oncology therapy, are essential for progressing therapies through clinical trials.
Securing these clearances represents significant value inflection points, enabling market access. For instance, a successful Phase 1 IND clearance in Q1 2024 allows critical trial progression.
- Critical IND clearances secured for multiple candidates.
- Potential Fast Track designations streamline development.
- Regulatory milestones enhance company valuation.
- Compliance ensures market readiness and patient access.
Vcanbio's core resources encompass its robust intellectual property in gene-editing and a proprietary cell bank that supports R&D. State-of-the-art cGMP-certified laboratories and a highly skilled team of scientists are vital physical and human capital. Crucial regulatory approvals, such as 2024 IND clearances, enable market progression and enhance company valuation. These assets collectively drive innovation and market access for advanced therapies.
| Resource Type | Specific Asset | 2024 Data Point |
|---|---|---|
| Intangible | Intellectual Property | Global gene-editing market >$11B |
| Physical | cGMP Facilities | Lab construction >$500/sq ft |
| Human | Expert Team | Biotech R&D spend >$250B |
| Intangible | Cell Bank | 15% increase in samples stored |
| Intangible | Regulatory Approvals | Phase 1 IND clearance Q1 2024 |
Value Propositions
Vcanbio offers profound hope, providing potentially curative treatments for debilitating conditions like advanced cancers and genetic disorders where conventional therapies have often fallen short. Our core value lies in transforming patient outcomes by leveraging cutting-edge cell and gene engineering, aiming to repair, replace, or regenerate damaged tissues and cells. The global cell and gene therapy market is projected to reach approximately $35 billion in 2024, underscoring the significant demand and investment in these innovative approaches. This focus on regenerative medicine provides a critical pathway to improved health and quality of life for those facing life-threatening illnesses.
For health-conscious individuals and families, we offer the unique security of banking their own healthy stem and immune cells. This personalized biological insurance policy preserves youthful, healthy cells for potential future use. With global investments in regenerative medicine projected to exceed $30 billion in 2024, this service aligns with cutting-edge advancements. It provides a proactive measure for future regenerative therapies, disease treatment, or anti-aging applications. This foresight addresses a growing demand for personalized health solutions, ensuring access to one's own optimal biological resources.
Vcanbio offers pharmaceutical and research partners critical access to its proprietary gene-editing and advanced cell therapy platforms. This enables partners to significantly accelerate their drug discovery and development programs, leveraging Vcanbio's specialized technology to tackle complex diseases without the immense capital expenditure of building such capabilities internally. For instance, the global gene therapy market is projected to reach over $20 billion in 2024, highlighting the increasing demand for outsourced platform expertise. This strategic partnership model allows companies to streamline R&D, focusing resources on specific therapeutic applications.
Integrated Health & Longevity Solutions
Vcanbio offers Integrated Health & Longevity Solutions, combining advanced cell banking with comprehensive genetic screening and personalized wellness consulting. This holistic approach empowers individuals to proactively manage their long-term health through data-driven biological insights. The global precision medicine market, a key component, is projected to exceed USD 175 billion by 2024, highlighting the demand for personalized health strategies.
- Personalized health plans based on genetic profiles.
- Long-term health management via cellular preservation.
- Proactive disease prevention strategies.
- Leveraging an estimated 2024 global genetic testing market value of over $20 billion.
De-risked & Validated Therapeutic Pipeline
Vcanbio offers investors and potential acquirers a portfolio of therapeutic candidates rigorously vetted through preclinical and early-stage clinical development. Our value proposition is a scientifically sound, de-risked pipeline with multiple shots-on-goal in high-value markets like oncology and rare diseases. As of 2024, early-stage oncology assets show an 8-10% success rate to approval, highlighting the value of de-risked programs. This focus mitigates investment risk by advancing programs past initial failure points. Our strategic pipeline focuses on areas with significant unmet medical need and market potential.
- Preclinical validation reduces R&D costs by an estimated 20% by avoiding later-stage failures.
- Early-stage clinical data, particularly Phase 1, boosts asset valuation significantly, often by 50-100% post-positive results.
- Targeting oncology and rare diseases aligns with markets projected for substantial growth, exceeding $300 billion by 2027 for oncology alone.
- A diversified pipeline minimizes single-asset dependency, enhancing overall portfolio resilience for investors.
Vcanbio provides potentially curative cell and gene therapies for severe conditions, operating within a global market projected at $35 billion in 2024. We empower individuals with personalized cell banking and integrated health solutions, aligning with a precision medicine market exceeding $175 billion by 2024. For partners, we offer access to advanced platforms, while investors benefit from our de-risked therapeutic pipeline targeting high-value markets like oncology. Our focus on innovation and market-aligned solutions drives significant value across healthcare stakeholders.
| Value Proposition | Key Benefit | 2024 Market Data |
|---|---|---|
| Curative Therapies | Transforming patient outcomes | Cell & Gene Therapy: ~$35B |
| Personalized Health | Future biological insurance | Regenerative Medicine: >$30B |
| Partner Platforms | Accelerated R&D via access | Gene Therapy: >$20B |
| Investor Pipeline | De-risked assets, high potential | Oncology Success Rate: 8-10% |
Customer Relationships
For Vcanbio's cell banking clients, relationships are built on profound trust, often spanning decades. This necessitates continuous communication and unwavering quality assurance, critical for long-term client retention. Clients receive annual status reports and secure online portal access, ensuring transparency and peace of mind, a key differentiator in a market where client retention rates for specialized services like cell banking can exceed 90% by 2024 due to high switching costs and trust. Maintaining this high-touch service is paramount for sustained engagement and loyalty.
Vcanbio establishes deep, supportive relationships with patients undergoing therapy, utilizing patient navigators and educational materials. This high-touch approach ensures adherence to complex treatments, which can boost patient compliance rates by over 30% in specialized therapies, as seen in 2024 clinical settings. Ongoing communication with physicians is crucial for managing these intricate regimens. This dedicated support also facilitates gathering critical long-term outcome data, essential for advancing cell and gene therapies.
Vcanbio fosters collaborative and transparent partnerships with pharmaceutical and research entities, managed through dedicated joint steering committees and alliance managers. This approach ensures strategic alignment and mutual success in co-development projects. Such partnerships are crucial, as evidenced by the projected global pharmaceutical R&D spending reaching approximately $275 billion in 2024, emphasizing the need for robust collaboration frameworks. Regular data sharing underpins these relationships, allowing for efficient progress tracking and problem-solving, enhancing the collective effort in bringing new therapies to market.
Scientific & Key Opinion Leader Engagement
Vcanbio strategically cultivates strong relationships with leading scientists and clinicians, referred to as Key Opinion Leaders (KOLs), within our specialized therapeutic fields. This vital engagement, crucial for credibility, is achieved through scientific advisory boards and collaborative research initiatives. Presenting at major medical conferences further solidifies our standing and drives adoption of our advanced technologies and therapies. Industry estimates for 2024 highlight that effective KOL engagement can increase market penetration by over 15% for new biotech products.
- Scientific advisory boards provide crucial clinical insights and validation.
- Collaborative research projects accelerate innovation and data generation.
- Medical conference presentations enhance visibility and peer recognition.
- KOL influence significantly impacts adoption rates of novel therapies.
Automated & Secure Digital Interaction
Vcanbio utilizes a secure, HIPAA-compliant digital platform for all routine interactions, serving both banking clients and clinical trial participants. This channel provides crucial self-service access for information, appointment scheduling, and data management, streamlining operations significantly. Such automation fosters an efficient and scalable relationship model, critical as digital health platform usage is projected to grow substantially in 2024. This approach also reinforces robust data privacy and security, aligning with stringent regulatory standards.
- Digital health platforms saw a 2024 market value exceeding $300 billion, emphasizing their widespread adoption.
- HIPAA compliance remains paramount, with data breaches costing healthcare organizations an average of $10.93 million per incident in 2024.
- Automated self-service channels can reduce customer service costs by up to 30% for financial and healthcare entities.
- Scalable digital solutions are essential, as clinical trial participation continues to expand globally in 2024.
Vcanbio cultivates deep, trust-based relationships with cell banking clients and patients, ensuring high retention and treatment adherence, with patient compliance rates boosting over 30% in 2024. Strategic partnerships with pharma and KOLs, crucial for market penetration over 15% by 2024, are managed through joint committees and advisory boards. A HIPAA-compliant digital platform, part of a $300+ billion 2024 market, streamlines interactions and reduces service costs by up to 30%, reinforcing data security and scalability.
| Relationship Type | Key Engagement | 2024 Impact Metric |
|---|---|---|
| Cell Banking Clients | Trust, quality assurance, transparency | 90%+ retention due to high switching costs |
| Patients | Navigators, education, ongoing communication | 30%+ boosted treatment compliance rates |
| Pharma & Research | Joint steering committees, data sharing | $275 billion global R&D spending |
| KOLs | Advisory boards, collaborative research | 15%+ market penetration for new products |
| Digital Platform | Self-service, secure data management | $300+ billion market value for digital health |
Channels
A specialized, highly-trained direct medical and scientific sales team is essential for Vcanbio. These professionals engage directly with hematologists, oncologists, and hospital administrators at major cancer centers across regions. This channel is crucial for educating medical professionals on complex cell therapies, which can significantly reduce the learning curve for new treatments. By fostering direct relationships, the sales force drives adoption in clinical settings, especially as new therapies receive regulatory approval, critical for market penetration and revenue growth in 2024 and beyond.
Vcanbio's dedicated business development team serves as a crucial channel, focusing on strategic partnerships and licensing with pharmaceutical and biotech companies. This team actively participates in major industry partnering conferences, such as BIO International, which in 2024 saw significant deal-making activity across the biotech sector. Through direct outreach, they aim to establish co-development, licensing, and commercialization agreements, essential for expanding market access and generating revenue. For instance, the value of biotech licensing deals globally reached over $200 billion in 2024, highlighting the importance of these channels for growth. These collaborations are vital for leveraging external innovation and maximizing product reach.
The company website, targeted social media campaigns, and search engine marketing form crucial digital channels for Vcanbio, aiming to reach prospective cell banking clients. This online presence, increasingly vital as global digital ad spending is projected to exceed $700 billion in 2024, delivers educational content and explains Vcanbio’s unique value proposition. These efforts are designed to generate high-quality leads for the consumer-facing aspects of the business. By leveraging platforms like LinkedIn and specialized health forums, Vcanbio can engage directly with individuals interested in advanced biotechnological services. This strategic digital outreach is key to expanding market penetration and enhancing brand recognition within the competitive biotech landscape.
Scientific Publications & Medical Conferences
Presenting research at prestigious scientific and medical conferences, such as the American Society of Gene and Cell Therapy annual meeting which saw over 7,000 attendees in 2024, is crucial for Vcanbio. Publishing findings in peer-reviewed journals, with over 30,000 biomedical journals active globally, validates their technology and builds significant credibility within the scientific and clinical communities. These channels are pivotal for influencing Key Opinion Leaders, who are essential for driving market acceptance and adoption of Vcanbio's stem cell and gene therapies.
- Vcanbio's presence at major 2024 biotech conferences enhances visibility.
- Peer-reviewed publications provide scientific validation for their therapeutic approaches.
- These channels directly influence clinical adoption and market perception.
- Engaging Key Opinion Leaders through research dissemination is paramount for market penetration.
Patient Advocacy Group Alliances
Partnering with patient advocacy groups, particularly for diseases like leukemia and lymphoma, creates a vital channel for Vcanbio to connect with and educate patient communities effectively. These alliances are crucial for accelerating clinical trial recruitment, a challenge where over 70% of trials face delays in 2024 due to enrollment issues. Such groups also amplify awareness of new therapeutic options, ensuring information reaches those who need it most.
Moreover, patient advocacy groups provide invaluable insights into patient needs and preferences, shaping Vcanbio's product development and support services. Their engagement can significantly enhance patient adherence and long-term outcomes, fostering trust and improving market penetration. In 2024, patient advocacy involvement in clinical trial design has grown by 15%, highlighting their increasing strategic importance.
- Patient advocacy groups facilitate direct patient community engagement and education.
- They are instrumental in improving clinical trial recruitment efficiency.
- These alliances significantly raise awareness for novel therapeutic options.
- PAGs offer critical insights into unmet patient needs and preferences.
Vcanbio uses direct sales and strategic partnerships, with biotech licensing deals exceeding $200 billion in 2024, for market penetration. Digital marketing, projected to exceed $700 billion in global ad spending in 2024, drives consumer leads. Scientific dissemination at conferences, like ASGCT 2024 with 7,000+ attendees, and patient advocacy groups, crucial as 70% of trials face enrollment delays, build credibility and access.
| Channel Type | Primary Function | 2024 Relevance |
|---|---|---|
| Direct Sales Teams | Clinical Adoption | Drives adoption in major cancer centers |
| Business Development | Strategic Partnerships | Biotech licensing deals over $200 billion |
| Digital Marketing | Consumer Lead Generation | Global digital ad spending over $700 billion |
| Scientific Forums | Credibility & Influence | ASGCT 2024 had over 7,000 attendees |
| Patient Advocacy Groups | Patient Engagement & Recruitment | 70% of trials face enrollment delays |
Customer Segments
Patients with critical diseases form Vcanbio's core customer segment, encompassing individuals diagnosed with specific cancers like leukemia or lymphoma, genetic disorders, and autoimmune conditions. These patients, the ultimate end-users of Vcanbio's innovative therapies, are primarily reached through established clinical trial sites and their treating physicians. For instance, in 2024, an estimated 61,090 new cases of leukemia were projected in the US alone, highlighting a significant patient population. Vcanbio's strategic focus ensures direct engagement with medical professionals to integrate new treatments into patient care pathways.
Hospitals and specialty treatment centers are primary customers, procuring and administering Vcanbio’s approved cell therapies to patients. Key decision-makers include heads of oncology departments, cell therapy lab directors, and hospital administrators. They critically evaluate clinical efficacy, cost-effectiveness, and logistical feasibility for integration. For instance, the global cell therapy market is projected to reach approximately $15 billion by 2024, emphasizing the significant procurement budgets these institutions manage. Their focus includes patient outcomes and the operational efficiency of new treatments within their existing infrastructure.
Health-conscious individuals and families, including new parents and affluent adults, form a key customer segment for Vcanbio's private stem cell and immune cell banking services.
Their primary motivation is securing future health options for themselves and their children, often driven by a proactive approach to potential medical needs.
The global cord blood banking market, valued at approximately $2.6 billion in 2023, is projected to grow, indicating a consistent demand from this segment.
In 2024, an estimated 700,000 new parents globally are considering private banking options, reflecting a strong market for long-term health preservation.
Pharmaceutical & Biotechnology Companies
Pharmaceutical and biotechnology companies represent a crucial B2B customer segment for Vcanbio, actively seeking to license Vcanbio's advanced gene-editing technology. These firms often partner on early-stage drug candidates to strategically fill gaps within their extensive pipelines. In 2024, global biopharmaceutical R&D spending continued its upward trajectory, with a significant portion allocated to external innovation and strategic alliances. This trend highlights the industry's reliance on specialized platforms like Vcanbio's to accelerate novel therapeutic development.
- Global pharma R&D expenditure reached over $240 billion in 2023, expected to grow in 2024.
- Gene editing market size was projected to exceed $7 billion in 2024, driven by therapeutic applications.
- Over 60% of new drug approvals in recent years involved external partnerships or licensing agreements.
- Biopharma companies are increasingly divesting non-core assets to focus on innovative, pipeline-enhancing technologies.
Academic & Government Research Institutions
Academic and government research institutions represent a key customer segment for Vcanbio, purchasing specialized research tools, proprietary cell lines, and contract research services. These entities, including major universities and government-funded laboratories, require access to cutting-edge biotechnologies to advance their foundational and translational research endeavors. Their demand is driven by the need for high-quality, reliable biological materials and advanced analytical capabilities to support diverse studies, from disease mechanisms to drug discovery. In 2024, global government R&D spending continued its upward trend, with significant allocations towards life sciences, bolstering demand for Vcanbio's offerings.
- Universities and government labs are primary customers for Vcanbio's research solutions.
- They procure specialized tools and cell lines for advanced research.
- Access to cutting-edge technology is essential for their basic and translational studies.
- Global government R&D funding in life sciences increased by an estimated 5% in 2024, supporting this segment.
Vcanbio's customer segments span patients with critical illnesses, hospitals administering advanced therapies, and health-conscious families utilizing private banking. It also serves pharmaceutical and biotech firms seeking gene-editing technology, alongside academic and government research institutions. This diverse approach targets both direct patient care and foundational biotechnological advancements for broad market impact.
| Segment | Key Offering | 2024 Market Data |
|---|---|---|
| Patients/Hospitals | Cell Therapies | Global Cell Therapy Market: ~$15B |
| Individuals/Families | Private Banking | New Parents Considering Banking: ~700,000 |
| Biopharma/Research | Gene Editing Tech/Tools | Gene Editing Market: >$7B |
Cost Structure
Research & Development expenses represent Vcanbio's primary cost driver, funding a large team of scientists, critical lab consumables, and extensive preclinical studies. This includes significant technology licensing fees, crucial for accessing cutting-edge innovations. The biotech sector's inherent high-risk, high-reward model demands substantial, ongoing R&D investment to sustain its innovation pipeline. For instance, top biotech firms often allocate over 30% of their revenue to R&D, a trend continuing into 2024 to fuel future therapeutic breakthroughs.
Conducting multi-center, multi-phase clinical trials is exceptionally expensive, with 2024 Phase 3 trials often exceeding $200 million per drug. Significant costs stem from patient recruitment, intricate data management, site monitoring, and extensive CRO services. Substantial funds are also allocated to preparing and filing complex regulatory submissions to bodies like the FDA, a process that can incur millions in fees and compliance overhead. These regulatory hurdles and trial complexities represent a major cost driver for Vcanbio.
Personnel and high-skilled labor costs are a significant operational expenditure for Vcanbio, primarily covering salaries, benefits, and stock-based compensation for its highly specialized workforce. This includes PhDs, MDs, and expert manufacturing personnel essential for biotech innovation. The intense competition for top talent in the global biotechnology sector, a market expected to reach over $1.7 trillion by 2024, consistently drives these human capital costs higher. Attracting and retaining these key resources is vital, as a skilled workforce directly impacts research, development, and production success.
Facility & Equipment Capital Expenditure
The construction and validation of Vcanbio's cGMP manufacturing facilities and advanced R&D laboratories represent a highly capital-intensive cost structure. This includes significant outlays for specialized equipment, often exceeding 10 million USD for a single biopharmaceutical production line in 2024, alongside ongoing depreciation. Utilities, stringent quality control protocols, and continuous maintenance are crucial for regulatory compliance and operational efficiency. For instance, maintaining ISO 14644 cleanroom standards requires substantial investment in HVAC and filtration systems.
- Capital expenditure for new cGMP facilities can reach hundreds of millions of USD.
- Annual maintenance and validation costs for such facilities often exceed 5% of their initial build cost.
- Depreciation of specialized biotech equipment significantly impacts the financial statements.
Sales, General & Administrative (SG&A)
Vcanbio's Sales, General & Administrative (SG&A) costs encompass essential operational expenses, including marketing initiatives, business development efforts, and critical legal fees for patent prosecution. This category also covers executive salaries, forming a significant fixed cost component. As Vcanbio progresses towards commercialization, sales and marketing expenses are projected to grow substantially, aiming to establish robust market access and ensure a successful product launch. For instance, biotech firms often allocate 20-30% of their pre-commercialization budget to SG&A.
- Marketing expenditures: Crucial for building brand awareness.
- Business development: Key for strategic partnerships.
- Legal fees: Essential for intellectual property protection in 2024.
- Executive salaries: Core to leadership and strategic direction.
Vcanbio's cost structure is dominated by substantial investments in Research & Development and expensive multi-phase clinical trials, often exceeding $200 million for 2024 Phase 3 trials. Significant capital expenditures are directed towards constructing cGMP facilities and acquiring specialized equipment, with a single production line often surpassing $10 million. High personnel costs for skilled scientists and a growing Sales, General & Administrative budget, potentially 20-30% of pre-commercialization spend, further define its operational expenses. This model demands continuous, substantial investment to drive innovation and achieve market entry.
| Cost Category | 2024 Data Point | Impact |
|---|---|---|
| R&D Allocation | Over 30% of revenue for top firms | Primary innovation driver |
| Phase 3 Clinical Trial Cost | Often exceeds $200 million per drug | Major drug development expense |
| Biotech Market Size | Expected over $1.7 trillion | Drives high talent competition |
| Single Production Line Cost | Over $10 million USD | High capital expenditure |
| SG&A Pre-Commercialization | 20-30% of budget | Essential for market access |
Revenue Streams
Cell and tissue banking fees represent a crucial recurring revenue stream for Vcanbio, generated from individual clients for the collection, processing, and long-term annual storage of stem cells and immune cells. This service provides a stable and predictable cash flow base, which is essential for funding ongoing research and development. For instance, the global cell banking market, including services like those offered by Vcanbio, was projected to continue its strong growth into 2024, emphasizing the consistent demand. This reliable revenue enables Vcanbio to strategically invest in and develop its therapeutic pipeline without solely relying on volatile R&D grants or equity financing.
Vcanbio generates significant revenue from strategic partnerships, especially with larger pharmaceutical companies, which is a key component of its business model. These agreements often include substantial upfront payments received upon signing the deal, providing immediate capital. Further income is generated through milestone payments, such as those achieved when clinical trials progress or specific R&D objectives are met, offering a structured revenue flow. Additionally, Vcanbio secures future royalties on the sales of co-developed products, ensuring long-term revenue streams from successful innovations. For instance, such partnerships contributed to the company's robust financial outlook for 2024, emphasizing this revenue channel's importance.
Upon securing regulatory approvals, the direct sale of Vcanbio's cell therapy products to hospitals and clinics will emerge as the primary revenue stream. These are high-value therapies, often commanding prices upwards of $400,000 per treatment, addressing critical diseases. For instance, in 2024, the market for advanced cell therapies continues to see products like CAR-T therapies priced in this range, reflecting their significant clinical impact. This revenue model leverages the substantial investment in R&D, converting approved treatments into significant commercial sales.
Technology Licensing & Royalty Fees
Vcanbio can generate significant revenue by licensing its proprietary gene-editing platforms and other advanced technologies to external biotech and pharmaceutical firms for their research and development programs.
This strategy provides crucial non-dilutive capital, complementing traditional funding, and effectively monetizes Vcanbio's intellectual property portfolio beyond its direct therapeutic products. In 2024, biotech licensing deals often include upfront payments, annual fees, and tiered royalties, which can range from 3% to 15% of net sales for successful therapies derived from licensed IP.
- Upfront payments and milestones secure early capital.
- Royalty rates typically range from 3% to 15% of product sales.
- Access to cutting-edge gene-editing platforms is highly sought after.
- Non-dilutive capital strengthens financial stability.
Contract Research & Manufacturing Services
Vcanbio leverages its specialized facilities and expertise by offering contract development and manufacturing organization (CDMO) services. This provides a crucial ancillary revenue stream, particularly for smaller biotech firms lacking their own advanced capabilities. By doing so, Vcanbio maximizes the utilization of its capital-intensive infrastructure. The global gene therapy market, a segment where such services are vital, was valued at USD 7.74 billion in 2023 and is projected to grow significantly from 2024 onwards.
- Vcanbio offers CDMO services to biotech firms.
- This maximizes facility utilization and generates ancillary revenue.
- Specialized facilities and expertise are key assets.
- The global gene therapy market was valued at USD 7.74 billion in 2023.
Vcanbio generates diverse revenue from stable cell and tissue banking fees, ensuring predictable cash flow. Direct sales of high-value cell therapies, some priced over $400,000, will become primary post-approval. Strategic partnerships yield upfront payments and future royalties, while licensing proprietary technology provides non-dilutive capital, with royalties often 3%-15% of net sales in 2024. Ancillary income comes from CDMO services, leveraging facilities in a gene therapy market valued at USD 7.74 billion in 2023 and growing significantly from 2024.
| Revenue Stream | Primary Mechanism | 2024 Data/Impact |
|---|---|---|
| Cell Banking | Annual storage fees | Consistent global market growth |
| Direct Therapy Sales | Per-treatment sales | Therapies exceed $400,000 |
| Licensing | IP usage fees | Royalties 3%-15% of net sales |
| CDMO Services | Contract manufacturing | Gene therapy market > $7.74B (2023) |
Business Model Canvas Data Sources
The Vcanbio Business Model Canvas is built upon a foundation of comprehensive market research, internal financial data, and analysis of competitive landscapes. These diverse data sources ensure that each component of the canvas accurately reflects Vcanbio's strategic positioning and operational realities.