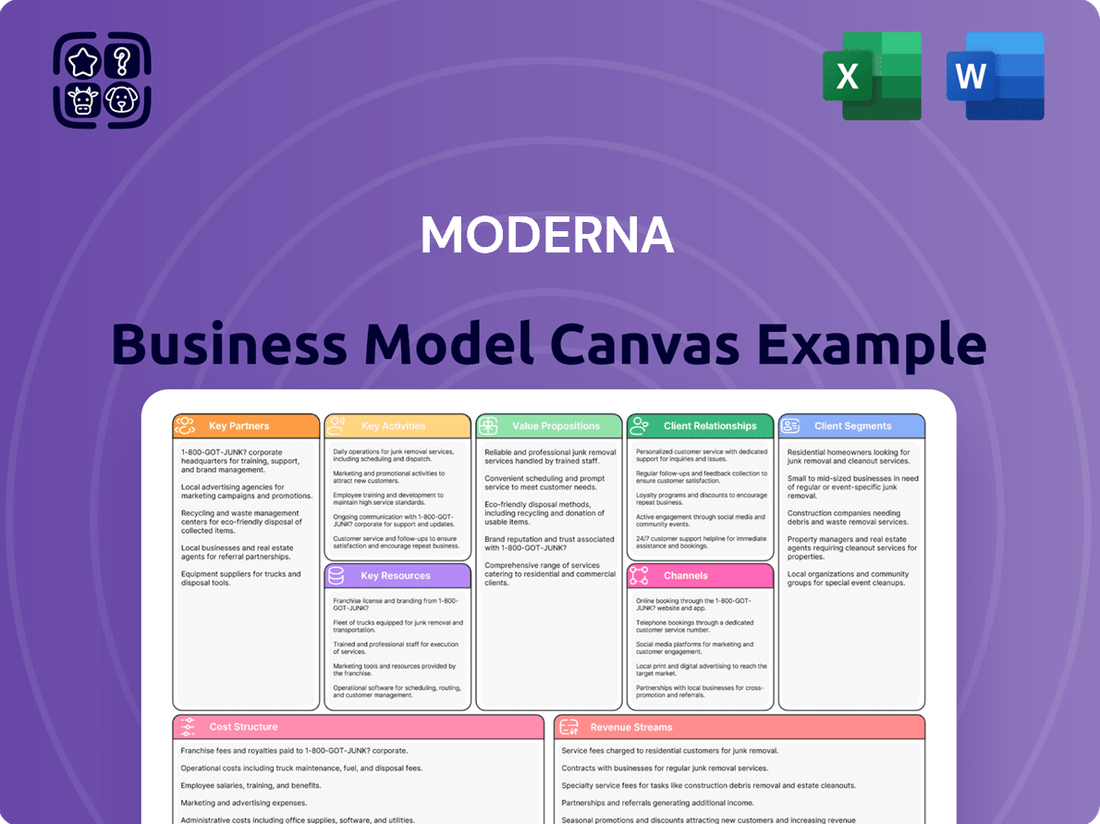
Moderna Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Moderna Bundle
Unlock the full strategic blueprint behind Moderna's business model. This in-depth Business Model Canvas reveals how the company drives value, captures market share, and stays ahead in a competitive landscape. Ideal for entrepreneurs, consultants, and investors looking for actionable insights.
Partnerships
Moderna actively pursues strategic alliances with other pharmaceutical and biotech firms. These collaborations are crucial for co-developing and bringing its innovative mRNA therapies to market.
A prime example is Moderna's ongoing partnership with Merck, focused on creating personalized cancer vaccines. Such alliances grant Moderna access to complementary technologies and specialized expertise, significantly broadening its market reach and therapeutic impact.
In 2024, these strategic partnerships are instrumental in accelerating the clinical development pipeline and navigating complex regulatory landscapes. The shared investment and risk further bolster the commercial viability of novel mRNA-based treatments.
Moderna collaborates extensively with governments and public health organizations worldwide, crucial for securing large-scale vaccine orders and ensuring effective distribution. This partnership is particularly vital for public health initiatives and pandemic preparedness.
A prime example is Moderna's substantial contracts with numerous governments for its COVID-19 vaccines. These agreements have been instrumental in their global rollout, demonstrating the significant role these partnerships play in public health crises.
Further solidifying these ties, Moderna announced a significant 10-year agreement with the UK government in 2023. This landmark deal focuses on advancing mRNA research and development, alongside building domestic manufacturing capabilities, underscoring a long-term strategic alliance.
Moderna's business model hinges on strategic alliances with Contract Manufacturing Organizations (CMOs) to rapidly scale its mRNA vaccine production. These partnerships are essential for manufacturing both key components and the final drug product, ensuring timely delivery. For instance, Moderna has inked deals with companies like Lonza and Catalent to bolster its manufacturing capacity, a critical factor in meeting global demand, especially during public health emergencies.
Beyond CMOs, securing a robust supply chain for raw materials is paramount. Moderna partners with specialized suppliers to obtain the necessary lipids, nucleotides, and other critical inputs vital for its messenger RNA (mRNA) technology. These collaborations are not just about volume but also about quality assurance, guaranteeing the integrity of the mRNA platform. By 2024, the company continued to solidify these relationships, understanding that supply chain resilience is directly linked to production output and market responsiveness.
Academic and Research Institutions
Moderna actively collaborates with top-tier academic institutions and research centers globally. These partnerships are crucial for pushing the boundaries of mRNA science, supporting foundational research and early-stage preclinical studies. For instance, the company has ongoing collaborations with institutions like Harvard University and MIT, leveraging their expertise in molecular biology and immunology.
These academic ties are instrumental in identifying and validating novel therapeutic targets and potential vaccine candidates. By tapping into cutting-edge academic discoveries, Moderna can accelerate its pipeline development. For example, research stemming from these collaborations has informed the company's work on infectious diseases and oncology.
In 2023, Moderna continued to strengthen its academic network, announcing new research agreements focused on areas such as rare diseases and autoimmune disorders. These collaborations often involve shared research efforts, data exchange, and access to specialized scientific knowledge, which are vital for innovation in the rapidly evolving mRNA field.
The impact of these partnerships is evident in Moderna's robust pipeline, which includes numerous candidates originating from or advanced through academic collaborations. These relationships not only contribute to scientific advancement but also provide a critical talent pool and foster a culture of continuous learning within the company.
- Foundational Research: Collaborations with universities like Yale and Stanford support fundamental understanding of mRNA delivery and immunogenicity.
- Preclinical Studies: Partnerships with research institutes such as the Broad Institute aid in validating novel mRNA constructs and targets.
- Therapeutic Target Discovery: Academic alliances help identify new disease pathways amenable to mRNA-based therapies.
- Advancing mRNA Science: Joint research efforts contribute to the broader scientific community's knowledge base regarding mRNA technology.
Healthcare Providers and Distributors
Moderna’s key partnerships with healthcare providers and distributors are crucial for getting its innovative mRNA vaccines and therapeutics to the people who need them. These collaborations ensure that products are not only manufactured but also delivered efficiently and administered safely. For instance, in 2024, Moderna continued its strong relationships with major pharmaceutical distributors. These include giants like McKesson Corporation, AmerisourceBergen, and Cardinal Health, which play a vital role in the complex supply chain of medical products. Their extensive networks allow for broad reach across various healthcare settings.
Beyond these large distributors, Moderna also engages in direct distribution to healthcare facilities in key markets. This direct approach can streamline the process, especially for specialized products or in regions where direct relationships offer greater control and responsiveness. This strategy is particularly important for managing the cold chain requirements of mRNA products, ensuring their efficacy from manufacturing site to patient. The company's ability to leverage these partnerships was a significant factor in its rapid scaling and product deployment, especially following the widespread demand for its COVID-19 vaccine.
- Distribution Network: Partnerships with McKesson Corporation, AmerisourceBergen, and Cardinal Health ensure broad access to Moderna's products.
- Direct Distribution: Direct engagement with healthcare facilities in key markets allows for tailored delivery and management.
- Supply Chain Integrity: These partnerships are essential for maintaining the quality and efficacy of temperature-sensitive mRNA products.
- Market Reach: Collaborations facilitate the efficient deployment of vaccines and therapeutics to diverse patient populations.
Moderna’s key partnerships with Contract Manufacturing Organizations (CMOs) are vital for scaling its mRNA vaccine production, with Lonza and Catalent being significant collaborators. These alliances ensure efficient manufacturing of both components and final products, meeting global demand, especially during health crises. By 2024, these relationships remained critical for production output and market responsiveness.
What is included in the product
A detailed breakdown of Moderna's approach, outlining its mRNA technology platform, key partnerships, and global distribution channels to deliver innovative vaccines and therapeutics.
The Moderna Business Model Canvas acts as a pain point reliever by streamlining complex vaccine development and distribution into a clear, actionable framework.
It simplifies the intricate journey from mRNA discovery to patient delivery, offering a digestible overview that addresses the challenges of rapid, large-scale biopharmaceutical innovation.
Activities
Moderna's central activity revolves around its extensive research and development efforts, with a strong focus on its innovative messenger RNA (mRNA) platform. This encompasses the entire drug development lifecycle, from initial discovery and preclinical testing to rigorous clinical trials. The company is committed to exploring a broad range of therapeutic areas, aiming to address unmet medical needs.
Currently, Moderna is actively progressing a robust pipeline of 45 distinct programs. These initiatives span critical areas such as vaccines for infectious diseases, a significant focus given its success with COVID-19, as well as promising therapies targeting cancer, rare diseases, and autoimmune conditions. This broad portfolio underscores Moderna's ambition to leverage its mRNA technology across diverse medical challenges.
Moderna's key activities center on the intricate manufacturing of its mRNA vaccines and therapeutics, encompassing the production of mRNA itself, the crucial lipid nanoparticles that deliver it, and the final formulated drug products. This complex process demands rigorous quality control and adherence to strict regulatory standards at every stage.
Managing a global supply chain is equally vital, ensuring that raw materials are sourced efficiently and finished products reach healthcare providers and patients worldwide in a timely manner. This logistical undertaking involves a sophisticated network of partners and distribution channels.
Moderna is actively investing in expanding its manufacturing footprint to meet growing global demand. Significant efforts are underway to increase capacity, with new facilities planned in strategic locations like the UK and Australia. These expansions are designed to bolster production capabilities, with the Australian facility, for instance, slated to begin producing vaccines in 2025.
Moderna's key activities heavily involve the meticulous execution of clinical trials, spanning Phase 1 through Phase 3, to rigorously assess the safety and effectiveness of its mRNA-based candidates. This scientific validation is paramount before any product can be considered for public use.
Equally crucial is the complex process of navigating global regulatory affairs. Securing approvals from bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) is a non-negotiable step to bring these innovative therapies and vaccines to market.
In 2024, Moderna continued its commitment to advancing its pipeline through these critical stages. For instance, the company was actively engaged in multiple Phase 3 trials for various indications, demonstrating significant investment in proving its technology's broad applicability and efficacy.
Commercialization and Marketing
Moderna actively commercializes its approved products, focusing on direct sales and targeted marketing campaigns. These efforts are crucial for reaching healthcare providers and public health officials, ensuring broad access to its innovative vaccines and therapeutics.
The company adapts its commercial strategies to changing market conditions. A prime example is the shift from the initial pandemic response to a more seasonal vaccine market, requiring adjustments in outreach and supply chain management.
In 2024, Moderna continued to refine its commercialization approach, particularly for its mRNA-based COVID-19 vaccines. This includes navigating payer landscapes and emphasizing the value proposition of its platform technology to diverse stakeholders.
Key commercialization and marketing activities for Moderna include:
- Direct Sales Force: Employing a dedicated sales team to engage with healthcare systems and large purchasers.
- Marketing Campaigns: Developing educational and promotional materials for healthcare professionals and the public.
- Public Health Engagement: Collaborating with government agencies and public health organizations to support vaccination programs.
- Market Access: Working to ensure reimbursement and formulary inclusion for its products.
Intellectual Property Management
Moderna's intellectual property management is a cornerstone of its business strategy. Protecting its proprietary mRNA technology, vaccine designs, and therapeutic formulations through patents and other IP mechanisms is a continuous and vital activity. This ensures Moderna maintains its competitive edge and secures future revenue streams.
- Patent Portfolio: Moderna actively manages a robust patent portfolio covering its mRNA platform, delivery systems, and specific vaccine candidates.
- Trade Secrets: Beyond patents, critical manufacturing processes and proprietary know-how are safeguarded as trade secrets.
- Licensing Agreements: Strategic licensing agreements are pursued to expand the reach of its technologies while generating revenue.
- R&D Investment Protection: IP protection is crucial for recouping significant investments in research and development, particularly for novel therapeutics.
Moderna's core activities involve pioneering mRNA technology for therapeutics and vaccines, encompassing discovery, preclinical, and clinical development across diverse diseases. The company actively pursues a broad pipeline, including 45 programs targeting infectious diseases, cancer, rare conditions, and autoimmune disorders, demonstrating a commitment to addressing significant unmet medical needs.
Manufacturing its mRNA-based products, including the mRNA itself and lipid nanoparticle delivery systems, is a critical operational focus, demanding high quality and regulatory compliance. Moderna is also strategically expanding its manufacturing capacity, with new facilities planned in locations like the UK and Australia to meet increasing global demand, with the Australian site expected to begin production in 2025.
Navigating global regulatory approvals, such as those from the FDA and EMA, is a vital step for bringing its innovative products to market. In 2024, the company actively managed multiple Phase 3 trials, underscoring its dedication to scientifically validating its platform's efficacy and broad applicability.
Commercialization efforts include direct sales, targeted marketing, and robust public health engagement to ensure broad access to its products. In 2024, Moderna refined its commercial strategies, particularly for its COVID-19 vaccines, adapting to market shifts and emphasizing its platform's value proposition to various stakeholders.
Intellectual property management, including a strong patent portfolio for its mRNA platform and proprietary processes, is fundamental to protecting its innovations and ensuring future revenue. Licensing agreements also play a role in expanding technology reach.
| Key Activity | Description | 2024 Focus/Data |
|---|---|---|
| Research & Development | Discovery and clinical trials of mRNA-based therapeutics and vaccines. | Actively progressing 45 distinct programs. Continued investment in Phase 3 trials across multiple indications. |
| Manufacturing | Production of mRNA, lipid nanoparticles, and final drug products. | Expanding manufacturing capacity globally; new facilities planned in UK and Australia. |
| Regulatory Affairs | Securing approvals from health authorities like FDA and EMA. | Ongoing submissions and engagement with regulatory bodies for pipeline assets. |
| Commercialization | Sales, marketing, and market access for approved products. | Refining COVID-19 vaccine commercialization strategies; emphasizing platform value. |
| Intellectual Property Management | Protecting mRNA technology through patents and trade secrets. | Active management of a robust patent portfolio; safeguarding proprietary manufacturing processes. |
What You See Is What You Get
Business Model Canvas
This preview offers a genuine glimpse into the comprehensive Moderna Business Model Canvas you will receive. It is not a simulation or a sample, but rather an exact representation of the document you'll gain access to upon purchase. When your order is finalized, you will download this identical, professionally structured Business Model Canvas, ready for your immediate use and customization.
Resources
Moderna's proprietary mRNA technology platform is the core of its business, acting as a sophisticated engine for creating novel therapeutics and vaccines. This platform facilitates the rapid design, research, and testing of a vast array of messenger RNA sequences, allowing for unparalleled agility in addressing diverse health challenges.
The platform's strength lies in its adaptability, enabling Moderna to pivot quickly in response to emerging diseases and scientific discoveries. This flexibility is crucial for maintaining a competitive edge in the fast-evolving biotechnology landscape. For instance, Moderna’s COVID-19 vaccine development timeline, from sequence identification to clinical trial readiness, was significantly accelerated by this technology.
In 2023, Moderna continued to invest heavily in its mRNA platform, with research and development expenses totaling approximately $5.0 billion. This sustained investment underscores the company's commitment to expanding the platform’s capabilities and exploring new therapeutic areas beyond infectious diseases, such as oncology and autoimmune disorders.
Moderna's intellectual property, primarily its extensive patent portfolio, is a cornerstone of its business model. These patents safeguard its proprietary mRNA technology, individual vaccine and therapeutic candidates, and unique manufacturing methods. This robust IP protection is vital for securing its competitive advantage and fostering continued innovation in the rapidly evolving biotechnology sector.
Moderna's scientific talent is its bedrock, a powerhouse of researchers and developers critical for its groundbreaking work. This expertise is not just about numbers; it's about the deep understanding of mRNA biology and immunology that fuels their innovative pipeline.
In 2024, Moderna continued to invest heavily in its scientific workforce, recognizing that a highly skilled team is paramount for navigating the complexities of drug development. Their success in bringing mRNA vaccines to market underscores the value of this human capital.
The company's ability to translate cutting-edge research into viable therapies hinges on the collective knowledge of its scientists, from early-stage discovery to clinical trials. This deep bench of expertise is a significant competitive advantage.
This scientific acumen is directly reflected in their robust pipeline and the ongoing development of new mRNA-based therapeutics for a range of diseases, a testament to the talent they cultivate and retain.
Manufacturing Facilities and Infrastructure
Moderna's manufacturing prowess is built on a hybrid model, leveraging both its owned facilities and a robust network of contract manufacturing organizations (CMOs) to ensure the scalable production of its mRNA vaccines and therapeutics. This flexibility allows for rapid expansion and diversification of supply chains.
The company has made significant strategic investments to bolster its global manufacturing footprint. For instance, in 2024, Moderna continued to expand its operations, including ongoing development of its new manufacturing facility in the United Kingdom, a key component of its European expansion strategy. Additionally, its partnership with Lonza, a leading CMO, has been instrumental in scaling production, with Lonza producing drug substance for Moderna's COVID-19 vaccine.
These investments are crucial for meeting the growing demand for mRNA-based medicines. Moderna's commitment to increasing its capacity is evident in its ongoing efforts to build out its own advanced manufacturing sites. By mid-2024, the company was operating multiple facilities globally, capable of producing hundreds of millions of doses annually, with plans to further increase this capacity through strategic site development and partnerships.
- Owned Facilities: Moderna operates its own state-of-the-art manufacturing sites, providing direct control over production processes and quality.
- CMO Partnerships: Collaborations with CMOs like Lonza are vital for augmenting production capacity and ensuring supply chain resilience.
- Global Expansion: Investments in new sites, such as the UK facility, demonstrate a commitment to expanding global manufacturing capabilities and accessibility.
- Capacity Growth: By 2024, Moderna's infrastructure was designed to support the production of hundreds of millions of doses, with ongoing investments aimed at further scaling capacity.
Capital and Financial Reserves
Capital and financial reserves are the bedrock of Moderna's operations, enabling its ambitious biotechnology ventures. These resources are critical for funding the long, complex, and expensive processes of research and development, rigorous clinical trials across multiple phases, and the subsequent scaling of manufacturing to meet global demand. Furthermore, significant capital is required to successfully commercialize innovative therapies.
As of the end of 2024, Moderna reported robust financial health, holding approximately $9.5 billion in cash, cash equivalents, and investments. This substantial liquidity provides the company with the necessary financial firepower to navigate the inherent risks and long timelines associated with drug development and to capitalize on emerging opportunities in the rapidly evolving biotech landscape.
- Substantial Capital for R&D: Funding cutting-edge research into mRNA technology and novel therapeutics.
- Clinical Trial Funding: Covering the extensive costs associated with human testing and regulatory submissions.
- Manufacturing Scale-Up: Investing in infrastructure and processes to produce therapies at commercial scale.
- Commercialization Efforts: Supporting market access, sales, and marketing for approved products.
- Financial Strength: Approximately $9.5 billion in cash, cash equivalents, and investments at the close of 2024.
Moderna's mRNA technology platform is its core asset, enabling rapid development of vaccines and therapeutics. This adaptability proved crucial for its swift COVID-19 vaccine response. In 2023, R&D spending reached about $5.0 billion, highlighting continued platform investment for new disease areas.
Intellectual property, particularly its patent portfolio, is fundamental to Moderna's competitive edge, safeguarding its mRNA technology and product pipeline. This robust protection is key for fostering innovation in the dynamic biotech industry.
Moderna's scientific talent is its foundation, with a highly skilled workforce driving innovation from research to clinical trials. In 2024, continued investment in this human capital underscored its importance for navigating drug development complexities.
The company's manufacturing strategy employs a hybrid model, combining owned facilities with CMO partnerships like Lonza for scalable production. By mid-2024, its global facilities were equipped to produce hundreds of millions of doses annually, with expansion ongoing, including a new UK site.
Capital reserves are vital for Moderna's extensive R&D, clinical trials, and manufacturing scale-up. By the end of 2024, the company held approximately $9.5 billion in cash and investments, providing significant financial capacity for its development pipeline and commercialization efforts.
Value Propositions
Moderna's novel mRNA technology is a cornerstone of its business model, enabling unprecedented speed in developing vaccines and therapeutics. This platform allows for rapid iteration and design, significantly shortening the traditional drug development timeline.
The company's ability to quickly pivot and create mRNA-based candidates was dramatically demonstrated with its COVID-19 vaccine, which received emergency use authorization in December 2020, a remarkably fast turnaround. This agility is a critical factor in its competitive advantage.
For 2024, Moderna continues to leverage this platform for its pipeline, including candidates for respiratory syncytial virus (RSV) and influenza, aiming to bring innovative solutions to market faster than conventional methods. The speed translates into earlier revenue potential and market entry.
Moderna's expansive pipeline is a cornerstone of its business model, targeting critical unmet medical needs across a broad spectrum of diseases. This includes developing potential treatments for infectious diseases beyond COVID-19, various cancers, rare genetic disorders, and debilitating autoimmune conditions.
This diversification is crucial, as it moves Moderna beyond its initial success in respiratory vaccines and signals a long-term commitment to innovation. By addressing these varied health challenges, the company positions itself to make significant contributions to global health and secure future revenue streams.
In 2024, Moderna continued to advance several key pipeline candidates. For instance, their mRNA-3704 for non-small cell lung cancer showed promising early data, and their investigational vaccine for respiratory syncytial virus (RSV) continued its clinical development, aiming to capture a significant share of that market.
Moderna's mRNA platform shows a strong potential for high efficacy and safety. The company's mid- and late-stage pipeline has a demonstrated probability of success notably higher than the industry average, underscoring the platform's reliability.
The recent approval of Moderna's respiratory syncytial virus (RSV) vaccine, its first commercially approved product, serves as a key validation. This success, coupled with ongoing positive Phase 3 data for other candidates, reinforces the company's ability to deliver on its therapeutic promises.
Scalability and Manufacturing Agility
Moderna's mRNA platform is built for scalability, meaning they can ramp up production quickly. This agility is crucial, allowing them to adapt manufacturing processes to create new vaccines against emerging variants or entirely new pathogens. For example, their COVID-19 vaccine production demonstrated this capability, rapidly scaling from initial development to millions of doses. This modular approach ensures a resilient supply chain, vital during public health emergencies.
The inherent flexibility of mRNA technology translates directly into manufacturing agility. This allows Moderna to pivot production efficiently, a critical advantage in responding to rapidly evolving health threats. Their ability to quickly adjust to new viral strains or the emergence of novel diseases ensures a more robust and reliable supply of medical countermeasures when they are needed most.
This adaptability has significant implications for public health preparedness. By being able to quickly reconfigure manufacturing lines, Moderna can address urgent needs arising from unexpected outbreaks. This capability is a key component of their business model, providing a valuable asset in the global fight against infectious diseases.
- Modular mRNA technology: Facilitates rapid scale-up and adaptation for new variants or pathogens.
- Manufacturing Agility: Enables quick shifts in production to meet evolving public health needs.
- Resilient Supply Chain: Ensures consistent availability of vaccines during health crises.
- Rapid Response Capability: Crucial for addressing emergent infectious disease threats.
Contribution to Global Health Security
Moderna's commitment to global health security is a cornerstone of its business model, extending far beyond profit motives. The company actively develops vaccines for a broad spectrum of infectious diseases, aiming to create defenses against potential pandemics and widespread health threats. This proactive approach is crucial for anticipating and mitigating future health crises on a global scale.
A significant aspect of this contribution involves strategic collaborations for vaccine distribution. Moderna partners with international organizations and governments to ensure its life-saving vaccines reach populations worldwide, particularly in low- and middle-income countries. This focus on accessibility is vital for achieving equitable global health outcomes.
In 2024, Moderna continued to emphasize its role in pandemic preparedness. For instance, the company has been actively developing next-generation mRNA vaccines targeting a range of pathogens beyond COVID-19, including influenza and respiratory syncytial virus (RSV). This pipeline diversification directly bolsters global health security by providing tools against prevalent and emerging diseases.
Moderna's efforts in 2024 also highlighted its dedication to equitable access. Through agreements with organizations like the World Health Organization's COVAX facility and various national governments, Moderna has worked to make its vaccines available at affordable prices in underserved regions. This strategy is essential for building resilience against infectious diseases across all socioeconomic strata.
- Disease Prevention: Development of mRNA vaccines against a wide array of infectious diseases to preemptively combat global health threats.
- Global Accessibility: Partnerships and initiatives focused on ensuring vaccines are available and affordable in low- and middle-income countries.
- Pandemic Preparedness: Continued investment in R&D for next-generation vaccines, enhancing the world's readiness for future outbreaks.
- Collaborative Distribution: Working with international health bodies and governments to facilitate widespread and equitable vaccine rollout.
Moderna's value proposition centers on its pioneering mRNA technology, offering rapid development cycles and a broad pipeline targeting unmet medical needs. This platform's inherent scalability and manufacturing agility ensure rapid response to emerging health threats and resilient supply chains.
The company's commitment to global health security is reinforced by strategic partnerships for equitable vaccine distribution, making life-saving innovations accessible worldwide. In 2024, advancements in RSV and oncology candidates, alongside pandemic preparedness efforts, underscore its multifaceted value.
Customer Relationships
Moderna actively cultivates direct relationships with national governments and global public health organizations, such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC). These engagements are crucial for securing large-scale vaccine supply agreements and are foundational to pandemic preparedness strategies.
These vital connections often translate into multi-year contracts, solidifying partnerships that extend beyond immediate public health crises. For instance, in 2024, ongoing discussions and agreements with various nations continue to shape future vaccine development and distribution plans, building on the significant uptake seen in previous years.
Moderna cultivates professional relationships with healthcare providers, including hospitals, clinics, and pharmacies, primarily through its dedicated commercial teams. These teams are instrumental in disseminating vital product information and offering ongoing support, ensuring healthcare professionals are well-informed about Moderna's offerings.
Their efforts are crucial in streamlining the vaccine ordering and administration processes for these institutions, directly impacting accessibility. This proactive engagement aims to educate the medical community about the benefits and efficacy of Moderna's vaccines, fostering trust and encouraging widespread adoption.
In 2024, Moderna continued its focus on these relationships, particularly as demand for its mRNA vaccines remained significant, driven by ongoing public health needs and the development of updated formulations. The company's commercial strategy emphasizes partnership and education to maximize vaccination uptake.
Moderna's strategic partnerships with biopharmaceutical companies are formalized through comprehensive collaboration agreements. These agreements outline joint development, licensing, and commercialization strategies, fostering a B2B environment focused on shared scientific and commercial objectives.
These collaborations are crucial for advancing Moderna's mRNA platform. For instance, in 2024, partnerships continued to drive research into new therapeutic areas beyond COVID-19 vaccines, leveraging the combined expertise and resources of both Moderna and its partners.
The nature of these relationships is inherently B2B, built on mutual scientific discovery and the potential for significant commercial returns. By pooling resources and knowledge, these partnerships accelerate the translation of mRNA science into life-changing medicines.
Investor Relations and Shareholder Communication
Moderna prioritizes open and consistent communication with its investors and shareholders. This is achieved through a variety of channels designed to provide clarity on the company's financial health, scientific advancements, and overarching strategy.
- Regular Financial Reporting: Moderna adheres to stringent SEC filing requirements, providing quarterly (10-Q) and annual (10-K) reports that detail its financial performance, including revenue figures, expenses, and cash flow. For instance, in Q1 2024, the company reported total revenue of $163 million, showcasing its ongoing commercialization efforts.
- Earnings Calls and Webcasts: The company regularly hosts conference calls and webcasts following the release of its financial results. These events allow management to discuss key performance indicators, answer analyst questions, and provide forward-looking statements.
- Investor Presentations and Conferences: Moderna actively participates in industry conferences and hosts dedicated investor days. These platforms offer opportunities to delve deeper into pipeline updates, manufacturing capabilities, and long-term growth strategies, fostering a more informed investor base.
- Shareholder Meetings: Annual and special shareholder meetings serve as a formal forum for shareholders to vote on corporate matters, hear updates from leadership, and engage directly with the company's governance.
Patient Advocacy and Awareness Campaigns (Indirect)
Moderna's customer relationships extend beyond direct patient engagement to include influential outreach via patient advocacy and awareness campaigns. These initiatives are crucial for building trust and educating the public about the potential of mRNA technology, especially for diseases with limited treatment options. For instance, in 2024, Moderna continued its commitment to disease awareness, supporting programs that highlight unmet medical needs and the role innovative therapies can play.
- Disease Education: Moderna actively participates in or supports campaigns aimed at educating the public and healthcare professionals about specific diseases, such as infectious diseases and rare genetic conditions, where mRNA technology shows promise.
- Advocacy Group Partnerships: The company collaborates with various patient advocacy organizations, providing resources and information to amplify their messages and support their efforts in empowering patients and families.
- Building Trust: By fostering open dialogue and providing transparent information about its research and development, Moderna aims to build a foundation of trust within patient communities and the broader public.
- Market Access Support: These indirect efforts also contribute to market access by increasing understanding and acceptance of novel therapeutic approaches, which is vital for the long-term success of its products.
Moderna maintains diverse customer relationships, from direct government contracts for vaccine supply to professional engagement with healthcare providers. Strategic B2B partnerships with other biopharmaceutical companies are key for advancing its mRNA platform, while transparent investor communication builds financial confidence. The company also engages with patient advocacy groups to foster trust and educate the public on mRNA technology.
| Relationship Type | Key Engagement Channels | 2024 Focus/Data Points |
|---|---|---|
| Governments & Public Health Orgs | Large-scale supply agreements, pandemic preparedness strategies | Ongoing discussions for future vaccine development and distribution plans. |
| Healthcare Providers | Commercial teams, product information dissemination, ordering/administration support | Focus on educating medical community, maximizing vaccination uptake. |
| Biopharmaceutical Partners | Collaboration agreements, joint development, licensing | Driving research into new therapeutic areas beyond COVID-19 vaccines. |
| Investors & Shareholders | Financial reporting (10-Q, 10-K), earnings calls, investor presentations | Q1 2024 total revenue reported at $163 million. |
| Patient Advocacy & Public | Disease education campaigns, partnerships with advocacy groups | Supporting programs highlighting unmet medical needs and the role of mRNA therapies. |
Channels
Moderna employs direct sales to governments for critical public health initiatives, such as national immunization campaigns. This channel was pivotal during the COVID-19 pandemic, enabling the efficient distribution of its mRNA vaccine on a large scale.
The company also engages with international organizations, notably COVAX, a global initiative aimed at equitable vaccine access. This direct engagement facilitates bulk purchasing and deployment across a wide range of countries, addressing global health needs.
For example, in 2021, Moderna secured significant supply agreements with the U.S. government, amounting to hundreds of millions of doses. These direct transactions streamline the procurement process, ensuring timely delivery for mass vaccination efforts.
This direct sales approach allows Moderna to establish predictable revenue streams and build strong relationships with key governmental and intergovernmental health agencies, crucial for long-term planning and market penetration.
Moderna leverages established pharmaceutical distributors like McKesson, AmerisourceBergen, and Cardinal Health to ensure broad market access for its innovative mRNA therapies. These partnerships are crucial for reaching a vast network of pharmacies, hospitals, and clinics across the United States and globally.
In 2023, these major distributors collectively handled a significant portion of the U.S. pharmaceutical market. For instance, McKesson reported net sales of approximately $239 billion for its fiscal year ending March 31, 2024, underscoring their extensive logistical capabilities and reach.
Through these alliances, Moderna can efficiently deliver its vaccines and therapeutics, ensuring they reach patients and healthcare providers promptly. This distribution infrastructure is vital for scaling up production and responding to public health needs, as demonstrated during the COVID-19 pandemic.
Moderna's mRNA therapeutics and vaccines reach patients through robust partnerships with established healthcare systems. These include major hospital networks, community clinics, and large retail pharmacy chains, ensuring broad access for vaccination campaigns and therapeutic treatments. For instance, during the COVID-19 pandemic, Moderna collaborated with the U.S. government and numerous pharmacy partners like CVS and Walgreens to facilitate widespread distribution and administration of its vaccine.
These established channels are critical for Moderna's go-to-market strategy, enabling efficient delivery and administration of its innovative products. By leveraging existing infrastructure, Moderna can quickly scale its operations and reach diverse patient populations. In 2024, the ongoing demand for its respiratory vaccines, including those for COVID-19 and influenza, continues to rely heavily on these established pharmacy and healthcare provider networks for patient access.
Company Website and Digital Platforms
Moderna's official website and digital platforms are crucial for disseminating scientific advancements, financial performance, and corporate news. These channels provide a direct link to a broad audience, from individual investors to healthcare professionals, offering detailed insights into their mRNA platform and pipeline. In 2024, the company continued to leverage these digital assets to communicate its strategic direction and R&D progress, reinforcing its commitment to transparency and stakeholder engagement.
- Scientific Communication: The website serves as a repository for clinical trial data, scientific publications, and updates on vaccine development, ensuring that the latest research is readily available.
- Investor Relations: Digital platforms host quarterly earnings reports, SEC filings, and investor presentations, providing essential financial data and strategic outlooks.
- Public Engagement: Moderna utilizes its digital presence to educate the public about mRNA technology and its potential applications beyond infectious diseases, fostering broader understanding and trust.
- Corporate Branding: These platforms are central to shaping Moderna's brand identity, highlighting its mission, values, and commitment to innovation in biotechnology.
Global Commercial Sales and Marketing Organization
Moderna has established a robust global commercial sales and marketing organization to ensure broad access to its groundbreaking mRNA therapies beyond the United States. This international infrastructure is crucial for navigating the complexities of diverse healthcare systems and regulatory environments worldwide.
This organization is tasked with more than just distribution; it's about strategic market penetration. They work to build relationships with healthcare providers, payers, and governments to facilitate the adoption and accessibility of Moderna's products. By understanding local needs and challenges, they tailor their approach for maximum impact.
- International Reach: Moderna's commercial presence extends to numerous countries, aiming for widespread availability of its vaccines and therapeutics.
- Regulatory Navigation: The team is adept at managing the intricate regulatory approval processes in each target market, a critical step for market entry.
- Market Access Strategies: Developing and executing tailored strategies to ensure affordability and accessibility for patients, working with local health authorities and payers.
- Commercial Partnerships: Forging collaborations with local partners, when appropriate, to enhance distribution networks and market understanding.
Moderna's channels are multifaceted, encompassing direct sales to governments for public health initiatives and partnerships with major pharmaceutical distributors like McKesson for broad market access. They also engage with established healthcare systems, including hospital networks and pharmacies, ensuring widespread patient reach. Furthermore, their digital platforms serve as vital hubs for scientific communication, investor relations, and public engagement, reinforcing brand identity and transparency.
Customer Segments
National governments and public health agencies are crucial customers for Moderna, particularly for bulk purchases of vaccines designed to protect entire populations. These entities prioritize widespread immunity and readiness for public health crises. In 2024, for instance, many nations continued to invest in vaccine stockpiles and ongoing vaccination programs, driven by the persistent threat of infectious diseases and a commitment to national health security.
Hospitals, clinics, and physician practices are critical customer segments for Moderna, as these entities directly administer vaccines and prescribe therapeutics. Their primary concerns revolve around a product's proven efficacy, robust safety profile, and practical aspects like ease of administration and seamless integration into existing clinical workflows. For instance, in 2023, hospitals continued to prioritize vaccines that demonstrated high efficacy against circulating strains of influenza and RSV, with physician adoption often tied to real-world effectiveness data.
Other biopharmaceutical companies are crucial partners for Moderna, seeking to integrate its cutting-edge mRNA technology into their own research and development pipelines. These collaborations are typically structured through licensing agreements, allowing partners to utilize Moderna's platform for specific therapeutic areas or disease targets.
Co-commercialization deals are also a common arrangement, where partners share the responsibilities and rewards of bringing new mRNA-based therapies to market. For instance, in 2024, Moderna continued to expand its portfolio of partnerships, with a significant focus on oncology and rare diseases, leveraging its mRNA capabilities to address unmet medical needs.
These companies are driven by the desire to accelerate drug development, diversify their product offerings, and gain a competitive edge by accessing novel therapeutic modalities. By engaging with Moderna, they aim to enhance their innovation capacity and potentially bring life-changing treatments to patients faster.
Patients (Indirectly via Healthcare System)
Patients, while not directly paying for Moderna's products, are the core reason for their existence. Their unmet medical needs are the driving force behind the company's innovation in mRNA therapeutics. For instance, the significant global demand for COVID-19 vaccines, driven by patient health concerns, directly influenced Moderna's rapid scaling and market penetration. In 2023, Moderna continued to focus on expanding its pipeline to address a wide range of diseases, aiming to improve patient outcomes and quality of life.
Moderna's success is intrinsically linked to patient access, which is largely facilitated through healthcare systems and public health initiatives. The company's mRNA platform is being explored for conditions like cancer, autoimmune diseases, and rare genetic disorders, all of which represent significant patient populations with critical needs. As of late 2023, clinical trials were underway for several new indications, reflecting a commitment to broadening patient benefit.
- Ultimate Beneficiaries: Patients are the end-users who gain health improvements from Moderna's mRNA-based medicines.
- Driving Innovation: Patient needs and the burden of disease are key factors guiding Moderna's research and development priorities.
- Access Channels: Patients typically receive Moderna's treatments through healthcare providers, hospitals, and national health programs.
- Global Impact: The widespread adoption of Moderna's COVID-19 vaccine highlighted the critical role of patient health in driving market demand.
Global Health Organizations
Global health organizations like Gavi, the Vaccine Alliance, are crucial customers for Moderna, particularly for vaccine supply to low- and middle-income countries. These entities are deeply committed to health equity and ensuring broad access to essential medicines. Their procurement decisions are heavily influenced by affordability and the potential for widespread distribution, making them strategic partners for expanding vaccine reach.
In 2024, Gavi continued its mission to increase access to immunization in poor countries. For example, their COVAX Advance Market Commitment played a significant role in distributing COVID-19 vaccines globally. Organizations like Gavi often negotiate bulk purchase agreements, requiring manufacturers to demonstrate scalable production and cost-effectiveness to meet the needs of diverse populations.
- Key Focus: Global health equity and access to medicines.
- Primary Need: Affordable and widely distributable vaccines.
- Procurement Strategy: Bulk purchases and long-term supply agreements.
- Impact: Facilitating vaccine access in low- and middle-income countries.
Academic and research institutions represent a vital customer segment for Moderna, utilizing its mRNA technology for groundbreaking scientific inquiry and drug discovery. These entities are driven by the pursuit of novel therapeutic targets and a desire to advance medical knowledge. In 2024, significant funding continued to be allocated towards mRNA research, supporting studies in areas beyond infectious diseases, such as oncology and rare genetic disorders, demonstrating the broad applicability of Moderna's platform.
Cost Structure
Moderna's business is fundamentally driven by innovation, making Research and Development (R&D) a cornerstone of its cost structure. This involves substantial investment in the entire lifecycle of new therapies, from initial scientific exploration and laboratory experiments to rigorous preclinical testing and multi-phase clinical trials. These activities are inherently expensive and time-consuming.
In 2024, Moderna allocated approximately $4.5 billion to R&D, underscoring the critical role of scientific advancement in its strategy. The company is specifically prioritizing investments in its oncology pipeline, aiming to accelerate the development of new cancer treatments, while also maintaining momentum in other therapeutic areas.
Manufacturing and production costs are a significant component of Moderna's business model, encompassing raw materials, facility operations, personnel, and rigorous quality control for its mRNA vaccines and therapeutics. For instance, in 2023, the company reported cost of sales of $7.9 billion, reflecting substantial investment in these operational areas.
Moderna has actively worked on optimizing these expenses, a strategy that included resizing its manufacturing infrastructure to better align with fluctuating market demand for its COVID-19 vaccines. This approach aims to improve efficiency and cost-effectiveness as the company scales its production capabilities.
Selling, General, and Administrative (SG&A) expenses at Moderna encompass crucial functions like commercialization of its mRNA vaccines and therapeutics, marketing efforts, maintaining a dedicated sales force, and overall corporate overhead. These costs are essential for bringing innovative products to market and supporting the business. For 2024, Moderna has been focused on optimizing its operations, with a stated goal of achieving significant cost efficiencies, particularly within SG&A, to position the company for sustained growth. This strategic focus is designed to streamline operations and improve the company's financial performance moving forward.
Clinical Trial and Regulatory Costs
Moderna’s business model incurs significant expenses for its robust clinical trial programs, which are essential for bringing new mRNA therapies to market. These costs encompass everything from recruiting and managing participants across multiple global sites to meticulously collecting and analyzing vast amounts of trial data. For instance, the development of their COVID-19 vaccine, while highly successful, involved extensive and costly trials involving tens of thousands of participants.
Beyond the trial execution itself, regulatory affairs represent another major expenditure. Moderna must pay substantial fees for submitting applications to regulatory bodies like the FDA and EMA, and ongoing compliance with evolving regulations adds to this cost. In 2023, Moderna reported research and development expenses of approximately $4.8 billion, a significant portion of which is directly attributable to clinical trial and regulatory activities for its pipeline of mRNA vaccines and therapeutics.
- Global Clinical Trial Operations: Costs associated with patient recruitment, site selection and management, investigator fees, and data monitoring across diverse geographical locations.
- Regulatory Submission and Approval: Fees paid to health authorities worldwide for the review and approval of new drug applications, as well as ongoing post-market surveillance and compliance.
- Pharmacovigilance and Safety Monitoring: Expenses related to the continuous monitoring of drug safety and efficacy once a product is on the market, including adverse event reporting and analysis.
- Manufacturing Scale-Up for Trials: Costs incurred in producing sufficient quantities of investigational medicinal products under strict Good Manufacturing Practice (GMP) standards for clinical trial use.
Intellectual Property and Legal Costs
Moderna's intellectual property and legal costs are substantial, reflecting the critical need to protect its mRNA technology and drug pipeline. These expenses cover patent applications, global filings, and ongoing maintenance fees to secure its innovations. In 2023, Moderna's total operating expenses were $7.8 billion, a significant portion of which is allocated to R&D and, by extension, IP protection.
- Patent Filings and Maintenance: Ongoing costs for filing new patent applications and maintaining existing ones globally, safeguarding its foundational mRNA platform and specific drug candidates.
- Legal Defense: Expenses incurred in defending its intellectual property against challenges and potential infringements, ensuring its competitive edge.
- Licensing and Agreements: Costs associated with managing and defending intellectual property rights within various licensing and collaboration agreements.
- Regulatory Compliance: While not solely legal, significant legal expertise is often required to navigate complex regulatory landscapes and ensure compliance, impacting overall legal expenditure.
Moderna's cost structure is heavily weighted towards research and development, with significant investments in clinical trials and manufacturing. The company's commitment to innovation necessitates substantial upfront spending on scientific exploration and product development. In 2024, R&D spending was projected at around $4.5 billion, highlighting the priority placed on advancing its mRNA pipeline.
Manufacturing and operational expenses, including cost of sales, were $7.9 billion in 2023, reflecting the complexity and scale of producing its advanced therapies. Selling, General, and Administrative (SG&A) costs are also significant, supporting commercialization and corporate functions, with a strategic focus on optimizing these in 2024 for improved efficiency.
Intellectual property protection and legal costs are crucial for safeguarding Moderna's proprietary mRNA technology, with total operating expenses reaching $7.8 billion in 2023. These expenditures are vital for maintaining its competitive advantage and ensuring the long-term viability of its innovative platform.
| Cost Category | 2023 Figures (Approximate) | Key Drivers |
| Research & Development (R&D) | $4.8 billion | New therapy discovery, preclinical testing, clinical trials |
| Cost of Sales (Manufacturing) | $7.9 billion | Raw materials, facility operations, quality control, production scale-up |
| Selling, General, & Administrative (SG&A) | Included within operating expenses, focus on optimization in 2024 | Commercialization, marketing, sales force, corporate overhead |
| Intellectual Property & Legal | Integral part of operating expenses ($7.8 billion total operating expenses in 2023) | Patent filings, legal defense, licensing agreements |
Revenue Streams
Moderna's core revenue generation stems from the sales of its successful vaccines and therapeutics. The flagship COVID-19 vaccine, Spikevax®, has been a significant contributor, alongside the newly approved respiratory syncytial virus (RSV) vaccine, mRESVIA®.
For the full year 2024, Moderna projected its total product sales to be in the range of $3.0 billion to $3.1 billion. Looking ahead to 2025, the company anticipated product sales to fall between $1.5 billion and $2.5 billion, reflecting evolving market dynamics for its COVID-19 vaccine and the initial ramp-up of mRESVIA®.
Moderna's revenue is significantly boosted by contracts with national governments for vaccine supply, often secured through advance purchase agreements that guarantee large volume orders. These agreements were particularly crucial during the COVID-19 pandemic.
For instance, in 2023, Moderna reported total revenue of $7.89 billion, with a substantial portion stemming from these government collaborations. While specific breakdowns for individual contracts are not always public, the company's historical reliance on such deals for its mRNA vaccines highlights their importance.
These government contracts provide a predictable revenue stream and allow Moderna to scale manufacturing efficiently, anticipating demand for public health needs. The advance purchase structure de-risks development and production for the company.
Moderna generates significant revenue through strategic collaborations and licensing agreements. These partnerships often involve upfront payments from collaborators, providing immediate capital. For instance, in 2024, such deals continue to be a cornerstone of their financial strategy, building on the success of prior agreements.
Milestone payments represent another crucial component of this revenue stream. As partners achieve specific developmental or regulatory targets with Moderna's mRNA technology, further payments are triggered, incentivizing progress and providing consistent financial inflows. This model was evident in the ongoing development of various vaccine candidates throughout 2024.
Furthermore, royalties on the future sales of licensed products form a long-term revenue driver. Once products developed through these collaborations reach the market, Moderna receives a percentage of the sales, creating a sustainable income source that benefits from the commercial success of its partners' endeavors.
These collaborations not only diversify Moderna's income but also leverage the expertise and market access of its partners, accelerating the development and commercialization of its innovative mRNA therapies and vaccines. This strategy was particularly apparent in the expansion of its pipeline discussions in 2024.
Grant Revenue
Moderna, like many biotechnology firms, supplements its core revenue streams with grants, particularly for early-stage research and development. These grants are typically awarded by governmental bodies, such as the National Institutes of Health (NIH), or private foundations supporting scientific advancement. While not a consistent or primary revenue source, grants play a crucial role in de-risking and funding novel therapeutic programs before they reach later, more commercially viable stages.
For instance, in 2024, Moderna continued to secure funding through various grant mechanisms to advance its pipeline. These awards often come with specific milestones and reporting requirements, tying the funding directly to progress in preclinical or early clinical studies. This type of non-dilutive funding is invaluable for exploration and innovation.
Key aspects of Moderna's grant revenue:
- Support for Novel Research: Grants often target groundbreaking areas, allowing Moderna to explore innovative technologies and therapeutic modalities that might not yet have a clear commercial path.
- De-risking Early-Stage Programs: Funding from grants can cover significant portions of early development costs, reducing the financial burden on the company and allowing for greater investment in a diverse portfolio.
- Partnerships with Public Health Initiatives: Some grants align with public health goals, enabling Moderna to contribute to the development of treatments or vaccines for diseases of significant societal impact.
- Diversification of Funding: While product sales are the main driver, grant revenue provides a supplementary and often non-dilutive source of capital, enhancing financial flexibility.
Future Therapeutic Sales
Future therapeutic sales represent a critical revenue stream for Moderna as its innovative mRNA platform matures and expands into new disease areas. As the company progresses its pipeline, especially in oncology and rare diseases, the commercialization of these novel treatments is anticipated to drive significant future revenue.
Moderna has set an ambitious target of achieving up to 10 product approvals by 2027, underscoring its commitment to broad therapeutic application. This expansion is crucial for diversifying revenue beyond its initial COVID-19 vaccine success and establishing a robust, multi-product commercial portfolio.
- Pipeline Expansion: Revenue will be generated from the commercial launch of mRNA-based therapies targeting oncology, rare diseases, autoimmune disorders, and other significant unmet medical needs.
- Product Approvals Target: The company aims for up to 10 product approvals by 2027, directly translating pipeline advancements into potential revenue-generating products.
- Market Penetration: Successful market entry and adoption of these future therapeutics will be key drivers of revenue growth, supported by clinical efficacy and value propositions.
- Diversification of Revenue: This stream is vital for reducing reliance on any single product and building a sustainable, long-term revenue base for Moderna.
Moderna's revenue streams are built on a foundation of vaccine and therapeutic sales, government contracts, strategic collaborations, and future product launches. The COVID-19 vaccine, Spikevax®, and the RSV vaccine, mRESVIA®, are current key products, with projected sales reflecting market shifts.
Government agreements, particularly advance purchase orders, have historically provided significant and predictable revenue, de-risking manufacturing scale-up. Collaborations and licensing deals contribute upfront payments, milestone achievements, and future royalties, broadening income sources and leveraging partner expertise.
Looking ahead, the company anticipates substantial revenue growth from its expanding pipeline of mRNA-based therapies in oncology, rare diseases, and autoimmune disorders, aiming for up to 10 product approvals by 2027 to diversify its commercial portfolio.
| Revenue Stream | Key Drivers | 2024 Projection (Product Sales) | Notes |
| Vaccine & Therapeutic Sales | Spikevax®, mRESVIA® | $3.0 - $3.1 billion | Reflects evolving COVID-19 vaccine market and mRESVIA® ramp-up. |
| Government Contracts | Advance purchase agreements | N/A (integrated into product sales) | Crucial for guaranteed volume and manufacturing scale. |
| Collaborations & Licensing | Upfront payments, milestones, royalties | N/A (contributes to overall revenue) | Diversifies income, leverages partner expertise. |
| Future Therapeutic Sales | Oncology, rare diseases, etc. pipeline | N/A (future growth driver) | Aiming for up to 10 product approvals by 2027. |
Business Model Canvas Data Sources
Moderna's Business Model Canvas is constructed using a blend of scientific research, clinical trial data, and intellectual property filings. These sources are crucial for defining its value proposition and key resources.
