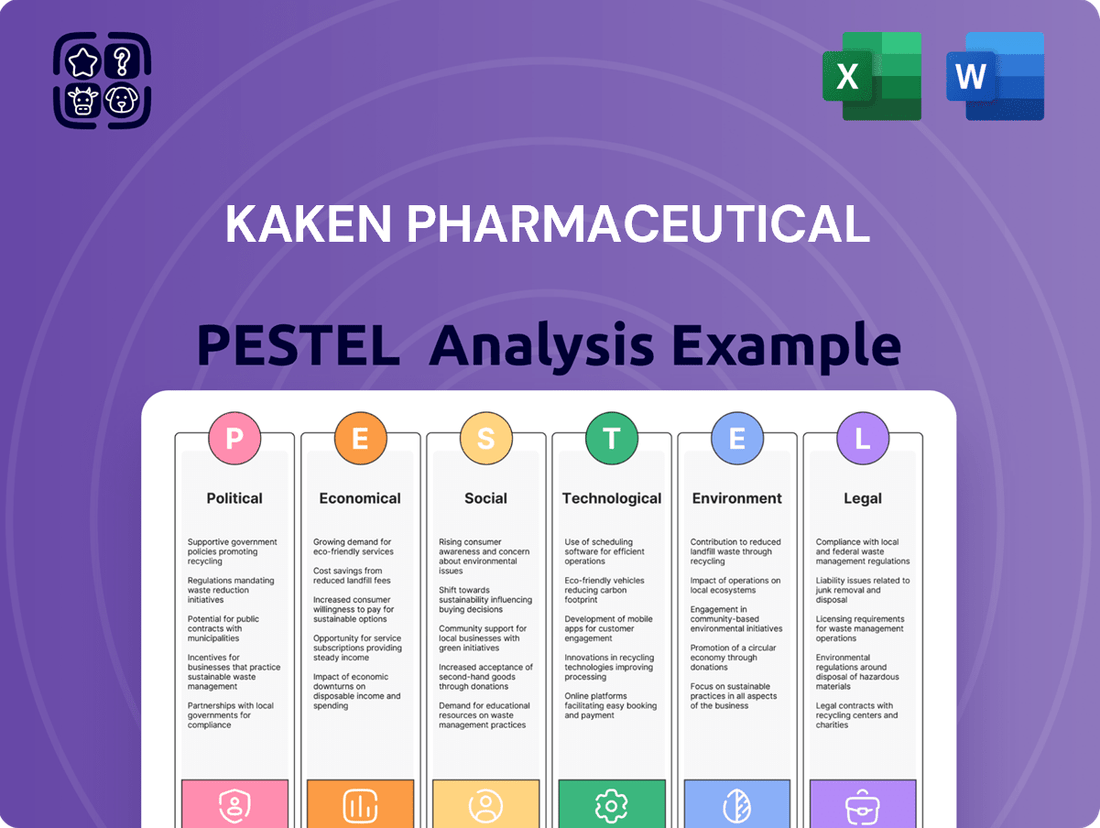
Kaken Pharmaceutical PESTLE Analysis
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Kaken Pharmaceutical Bundle
Gain a critical advantage by understanding the external forces shaping Kaken Pharmaceutical's trajectory. Our PESTLE analysis delves into political stability, economic shifts, evolving social attitudes, technological advancements, environmental regulations, and legal frameworks impacting the company. Don't just react to market changes; anticipate them.
Unlock actionable intelligence crucial for strategic planning and investment decisions. This comprehensive PESTLE analysis for Kaken Pharmaceutical offers a clear roadmap of opportunities and threats. Equip yourself with the insights needed to navigate the complex pharmaceutical landscape.
Ready to make informed decisions? Our expertly crafted PESTLE analysis of Kaken Pharmaceutical is your key to understanding the broader market dynamics. Download the full version now and transform raw data into strategic foresight.
Political factors
Government policies, especially in Japan, wield substantial influence over drug pricing and reimbursement structures. The Japanese Fiscal Year 2025 NHI drug price revision, effective from December 2024, directly impacts pharmaceutical revenues by implementing price reductions tied to discrepancies between existing NHI prices and actual market prices.
While mechanisms such as the Price Maintenance Premium (PMP) exist to offer some protection for innovative drugs against these reductions, the overarching trend indicates persistent pressure to manage drug costs as part of broader healthcare expenditure control efforts.
The speed and rigor of regulatory approvals in Kaken's primary markets significantly influence its new product launches. Japan’s initiative to increase drug price listing reviews from four to seven times annually, effective January 2025, signals a commitment to faster market access for novel therapies.
Kaken Pharmaceutical’s global footprint means it’s directly influenced by international trade agreements and tariffs. Changes in these policies can significantly affect its supply chain, from the cost of acquiring raw materials to the pricing of its finished pharmaceutical products in different markets. For instance, while the pharmaceutical sector has seen temporary exemptions from certain US import tariffs, ongoing volatility in global trade relations creates persistent uncertainty.
This trade uncertainty can indirectly dampen growth prospects for the entire pharmaceutical industry, including Kaken. For example, the World Trade Organization (WTO) reported a 1.9% decline in global merchandise trade volume in 2023, reflecting broader geopolitical tensions that can spill over into trade policy. Such an environment necessitates careful monitoring of trade pacts and tariff structures to mitigate potential financial impacts.
Political Stability and Geopolitical Risks
Political stability in key markets, including Japan and potential expansion regions, directly impacts Kaken Pharmaceutical's operational continuity and its ability to access new patient populations. Geopolitical risks, such as trade disputes or regional conflicts, can introduce significant unpredictability into the pharmaceutical supply chain and R&D investment decisions.
For instance, the ongoing geopolitical tensions in Eastern Europe, while not directly involving Kaken's core markets, contribute to a general increase in global economic uncertainty, potentially affecting investor confidence and capital availability for pharmaceutical ventures. Shifts in government healthcare policies, a common occurrence in the pharmaceutical sector, can alter reimbursement rates and market access for Kaken's products, as seen with recent discussions around drug pricing reforms in several major developed economies during 2024.
- Political Stability: Kaken operates in Japan, a highly stable political environment, but expansion into other regions necessitates careful assessment of local political risks.
- Geopolitical Risks: Global trade tensions and regional conflicts can disrupt Kaken's international supply chains and impact its market access strategies.
- Regulatory Environment: Changes in government healthcare priorities and pharmaceutical regulations in operating countries can significantly influence market dynamics and profitability.
- Government Support for R&D: Political decisions regarding funding for scientific research and development can create opportunities or challenges for Kaken's innovation pipeline.
Government Support for R&D and Innovation
Government support for research and development is a significant driver for pharmaceutical companies like Kaken. Japan's commitment to fostering pharmaceutical innovation is evident through various initiatives. For example, the government has introduced measures to incentivize early adoption of new drugs, such as expanding eligibility for usefulness premiums and creating new price premiums, specifically targeting the issue of drug lag.
These policies aim to accelerate the availability of novel treatments and encourage investment in cutting-edge research. In 2023, the Japanese government continued to emphasize support for life sciences, with ongoing discussions and policy adjustments designed to bolster the domestic pharmaceutical industry's competitiveness on a global scale. This focus translates into potential funding opportunities and a more favorable regulatory environment for R&D.
- Government Funding: Japan's Ministry of Health, Labour and Welfare (MHLW) actively supports pharmaceutical R&D through grants and subsidies.
- Incentive Programs: New pricing policies introduced in recent years aim to reward early market entry for innovative medicines, encouraging faster development cycles.
- Addressing Drug Lag: Specific policy reforms are in place to reduce the time it takes for new drugs approved overseas to become available in Japan, a key focus for 2024 and beyond.
- Innovation Ecosystem: Efforts are underway to strengthen the entire innovation ecosystem, from basic research to commercialization, fostering collaboration between academia and industry.
Government policies, particularly in Japan, significantly shape Kaken Pharmaceutical's revenue streams through drug pricing and reimbursement. The Japanese Fiscal Year 2025 NHI drug price revision, effective December 2024, implements price reductions based on market price deviations. While the Price Maintenance Premium offers some protection for novel drugs, the trend points to ongoing cost containment pressures within the healthcare system.
Regulatory approval timelines are crucial for Kaken's product launches, with Japan's move to increase drug price listing reviews to seven times annually from January 2025 aiming to expedite market access for new therapies.
International trade agreements and tariffs directly impact Kaken's global supply chain and product pricing, with ongoing trade volatility creating persistent market uncertainties. For instance, global merchandise trade volume saw a 1.9% decline in 2023, reflecting broader geopolitical tensions that can influence trade policies.
Political stability in Kaken's operating and expansion markets is essential for operational continuity and market access. Geopolitical risks, such as trade disputes, can disrupt supply chains and influence R&D investment decisions, contributing to overall economic uncertainty for the pharmaceutical sector.
What is included in the product
This PESTLE analysis of Kaken Pharmaceutical examines the Political, Economic, Social, Technological, Environmental, and Legal factors impacting its operations and strategic planning.
It provides a comprehensive overview of the external landscape, highlighting key trends and potential challenges to inform decision-making.
Kaken Pharmaceutical's PESTLE Analysis provides a pain point reliever by offering a clean, summarized version of external factors for easy referencing during meetings or presentations, streamlining strategic discussions.
Economic factors
Global healthcare spending is on an upward trajectory, with projections indicating continued growth. In 2023, worldwide health expenditure was estimated to be around $10 trillion, and this figure is expected to climb further in the coming years.
Japan's healthcare expenditure also reflects this trend, driven by its rapidly aging population, which naturally increases demand for medical services and pharmaceuticals. By 2025, Japan's healthcare spending is anticipated to reach approximately $460 billion.
This expanding healthcare market, both globally and specifically within Japan, directly impacts Kaken Pharmaceutical by influencing the overall size and growth potential of the markets where its products are sold.
The pharmaceutical sector, in particular, is a significant beneficiary of these spending increases, with the global pharmaceutical market forecast to reach over $2 trillion by 2028, and the Japanese market showing robust growth fueled by the need for advanced treatments.
Biennial drug price revisions, a common practice in Japan, can significantly disrupt revenue streams for pharmaceutical companies like Kaken. These scheduled adjustments, along with off-year revisions, create an environment of uncertainty, directly impacting profitability. The upcoming Japanese Fiscal Year 2025 NHI drug price revision and the parallel 2025 off-year revision are particularly concerning for the industry, with anticipated price cuts posing a substantial challenge.
Despite ongoing efforts to strike a balance between controlling healthcare costs and incentivizing pharmaceutical innovation, the prospect of further price reductions remains a key concern. This regulatory landscape necessitates robust financial planning and strategic adaptation to mitigate the potential negative impacts on Kaken's revenue and overall financial health. The cyclical nature of these price adjustments requires continuous monitoring and proactive management.
Currency exchange rate fluctuations present a significant economic factor for Kaken Pharmaceutical, given its global operations. When the Japanese Yen strengthens against other currencies, Kaken's overseas sales revenue, when converted back to Yen, becomes less valuable. Conversely, a weaker Yen can boost reported international earnings. This volatility directly influences the profitability of its international commercialization efforts.
For instance, in fiscal year 2024, the average exchange rate for the US Dollar was approximately 145 Yen, a notable depreciation from earlier periods. This depreciation would have generally benefited Kaken's reported earnings from its significant US market presence. However, if Kaken sources raw materials or conducts R&D in countries with stronger currencies, a weaker Yen would increase those costs in Yen terms, creating a balancing effect.
The company's financial statements often include disclosures detailing the impact of foreign currency translation. Analyzing these disclosures, particularly for the 2024 and projected 2025 fiscal years, reveals the sensitivity of Kaken's net income to movements in major currency pairs like USD/JPY and EUR/JPY. These fluctuations are a constant consideration in financial planning and risk management for Kaken.
Inflation and Economic Recession Risks
Inflationary pressures in 2024 and 2025 are a significant concern for Kaken Pharmaceutical. Rising costs for raw materials, energy, and labor can directly impact research and development budgets, potentially slowing innovation. For instance, the US Consumer Price Index (CPI) saw a notable increase in 2023, and while projections for 2024-2025 suggest a gradual moderation, persistent inflation remains a risk. This also translates to higher manufacturing expenses and general operational overheads, squeezing profit margins.
Economic recession risks, particularly in key markets like Japan and globally, could curtail healthcare spending. Governments might implement stricter reimbursement policies or reduce drug prices to manage public finances. While the pharmaceutical industry often demonstrates defensive characteristics, a prolonged downturn could dampen demand for Kaken's products, especially those in less critical therapeutic areas. For example, the IMF's outlook for global growth in 2024 has been subject to revision, highlighting the uncertainty.
- Increased R&D and Manufacturing Costs: Persistent inflation throughout 2024-2025 directly escalates the expenses associated with developing new drugs and producing existing ones.
- Potential for Reduced Healthcare Spending: Economic downturns can lead to tighter government budgets and decreased patient out-of-pocket spending on pharmaceuticals.
- Impact on Reimbursement Policies: Recessions often trigger governments to review and potentially reduce the reimbursement rates for pharmaceutical products.
- Demand Sensitivity: While essential medicines are somewhat insulated, demand for less critical treatments could soften during periods of economic contraction.
Investment in R&D and Market Dynamics
Kaken Pharmaceutical’s future growth is significantly influenced by its research and development (R&D) investment strategy and the ever-evolving market dynamics within the pharmaceutical sector. Competitors are also channeling substantial resources into R&D, especially in areas like personalized medicine and novel drug delivery systems. This intense focus on innovation is a critical factor for Kaken to maintain its competitive edge.
The broader pharmaceutical industry is experiencing robust R&D investment, with projections indicating continued strength through 2025. This trend is particularly evident in the development of targeted therapies, which offer more precise treatment outcomes. Furthermore, market dynamics, including mergers and acquisitions (M&A), are expected to accelerate in 2025, presenting both opportunities and challenges for Kaken.
- R&D Spending Trends: Global pharmaceutical R&D spending reached approximately $243 billion in 2023, with continued growth anticipated.
- M&A Outlook: Analysts predict an increase in pharmaceutical M&A activity in 2025, driven by the need for pipeline expansion and market consolidation.
- Focus Areas: Key R&D investment areas include oncology, immunology, and rare diseases, reflecting patient needs and commercial potential.
- Competitive Landscape: Kaken must continually assess competitor R&D pipelines and strategic alliances to inform its own investment decisions.
Japan's biennial drug price revisions, set for 2025, along with off-year adjustments, pose a significant economic challenge for Kaken Pharmaceutical, potentially impacting revenue and profitability due to anticipated price cuts. Global and domestic healthcare spending continues to rise, with Japan's expenditure projected to reach around $460 billion by 2025, driven by an aging population. However, currency fluctuations, particularly the Yen's strength against other currencies, can negatively affect Kaken's reported overseas earnings, while inflation in 2024-2025 escalates R&D and manufacturing costs.
Economic downturns in 2024-2025 could also lead to reduced healthcare spending and tighter government budgets, potentially impacting reimbursement policies and dampening demand for Kaken's products. The pharmaceutical sector's R&D spending is robust, with global expenditure around $243 billion in 2023, and M&A activity is expected to increase in 2025, creating a dynamic competitive landscape for Kaken.
| Economic Factor | Impact on Kaken Pharmaceutical | 2024-2025 Data/Projection |
| Drug Price Revisions (Japan) | Potential revenue reduction due to price cuts | Biennial revision in 2025, off-year revision also anticipated |
| Healthcare Spending Growth | Larger market size, increased demand | Japan spending projected at ~$460 billion by 2025; Global spending ~$10 trillion in 2023 |
| Currency Exchange Rates | Impact on international sales revenue (e.g., USD/JPY) | USD/JPY averaged ~145 in FY2024; fluctuations impact reported earnings and costs |
| Inflation | Increased R&D, manufacturing, and operational costs | Persistent inflation in 2024-2025; US CPI rose in 2023, gradual moderation projected |
| Economic Recession Risks | Potential reduction in healthcare spending, tighter budgets | IMF global growth outlook subject to revision; defensive sector but prolonged downturn impacts demand |
Preview Before You Purchase
Kaken Pharmaceutical PESTLE Analysis
The preview shown here is the exact document you’ll receive after purchase—fully formatted and ready to use. This comprehensive PESTLE analysis of Kaken Pharmaceutical delves into the Political, Economic, Social, Technological, Legal, and Environmental factors impacting the company's operations and strategic direction. It provides a detailed examination of current trends and potential future influences, offering valuable insights for informed decision-making.
Sociological factors
Japan's population is aging rapidly; by 2025, the proportion of citizens aged 65 and over is projected to reach nearly 30%, according to recent demographic forecasts. This significant demographic shift directly fuels a greater need for pharmaceutical solutions targeting age-related ailments, a core area for Kaken Pharmaceutical. The increasing prevalence of chronic conditions associated with aging, such as osteoarthritis and various skin disorders, presents a substantial market opportunity for Kaken's specialized treatments.
The rising incidence of chronic diseases, exacerbated by an aging demographic, translates into sustained demand for Kaken's product portfolio. For instance, the market for orthopedic drugs, a key segment for Kaken, is expected to see continued growth due to the higher likelihood of conditions like osteoporosis and joint pain in older individuals. This trend is a fundamental driver for Kaken's long-term revenue streams and strategic focus on developing and marketing treatments for these prevalent health concerns.
Shifting lifestyles, influenced by factors like urbanization and dietary changes, are altering disease prevalence, leading to an increase in chronic conditions alongside persistent infectious diseases. This evolution directly impacts Kaken Pharmaceutical's strategic focus, particularly in areas like dermatology and infectious disease treatments.
For instance, the growing concern over antibiotic resistance, a direct consequence of evolving treatment patterns and pathogen adaptation, necessitates ongoing research and development for novel anti-infective agents. Globally, the World Health Organization reported in 2023 that antimicrobial resistance (AMR) is a significant and growing threat, contributing to millions of deaths annually, a trend Kaken must actively address.
Furthermore, increased exposure to environmental factors and changing personal care habits are contributing to a rise in certain dermatological conditions. Kaken's dermatological products are thus positioned to benefit from this trend, provided they can innovate to meet evolving patient needs for conditions like eczema or acne, which are often linked to lifestyle changes.
Heightened public awareness surrounding health, amplified by events like the ongoing COVID-19 pandemic, is directly driving a greater demand for innovative pharmaceutical solutions. This trend benefits companies like Kaken Pharmaceutical, as patients and healthcare professionals actively seek out advanced treatments offering improved quality of life and better health outcomes.
In 2024, the global pharmaceutical market is projected to continue its growth trajectory, with a significant portion of this expansion attributed to the demand for novel therapies. Kaken's focus on areas like pain management and dermatology, where unmet patient needs remain high, positions it well to capitalize on this evolving landscape.
Healthcare Access and Affordability
Societal expectations for equitable healthcare access and affordable medicines significantly shape Kaken Pharmaceutical's market entry and pricing approaches. In Japan, the commitment to universal health coverage, a cornerstone of its social policy, directly influences drug price negotiations and distribution channels. This drive for affordability means Kaken must navigate a system where government reimbursement plays a crucial role in market success, impacting revenue potential for its innovative treatments.
The Japanese government's stance on healthcare affordability is evident in its drug pricing policies. For instance, the biannual drug price revisions aim to control pharmaceutical expenditure, a critical factor for companies like Kaken. As of late 2024, the focus remains on balancing innovation with cost containment, requiring Kaken to demonstrate clear therapeutic value and cost-effectiveness to secure favorable pricing and reimbursement status.
- Universal Health Coverage: Japan's system ensures all citizens have access to medical services, influencing demand and pricing strategies.
- Government Reimbursement: The Ministry of Health, Labour and Welfare's pricing decisions are paramount for Kaken's product viability.
- Cost-Effectiveness Demands: Kaken must prove its drugs offer superior value compared to existing treatments to justify higher price points.
- Aging Population: Japan's demographic trends increase demand for healthcare services, particularly for conditions prevalent in older adults, a key market for Kaken.
Ethical Considerations and Social Acceptance of New Therapies
Societal views on novel medical treatments, such as gene editing and advanced cell therapies, significantly shape their acceptance and the speed of regulatory approval. Kaken Pharmaceutical's focus on innovative solutions, including its work in cell therapy for orthopedic conditions, necessitates a deep understanding of public sentiment and established ethical guidelines to ensure successful market integration.
Public perception regarding the safety and efficacy of cutting-edge medical technologies plays a crucial role in their adoption. For instance, in 2024, surveys indicated that while a majority of the public expressed optimism about the potential of gene therapies, a notable percentage also voiced concerns about long-term side effects and equitable access, impacting Kaken's market entry strategies for its advanced treatments.
- Public Trust: A 2024 report by the Pew Research Center found that 65% of Americans are open to gene editing for treating serious diseases, but only 30% are comfortable with its use for enhancing traits. This duality requires Kaken to focus on clear communication about therapeutic benefits.
- Ethical Debates: Ongoing discussions around the ethics of cell manipulation and the potential for unintended consequences, such as off-target effects in gene editing, create a cautious environment that Kaken must navigate through transparent research and dialogue.
- Patient Advocacy: The increasing influence of patient advocacy groups, who champion access to new therapies, can accelerate acceptance. Kaken's engagement with these groups in 2025 will be vital for building social license and demonstrating commitment to patient well-being.
- Regulatory Scrutiny: Societal concerns often translate into stricter regulatory oversight. Agencies in major markets are closely monitoring the ethical implications of new therapies, meaning Kaken's research and development must adhere to the highest ethical standards to meet evolving compliance requirements.
Societal expectations for equitable healthcare access and affordable medicines significantly shape Kaken Pharmaceutical's market entry and pricing strategies. Japan's commitment to universal health coverage means Kaken must navigate a system where government reimbursement, influenced by biannual drug price revisions, is paramount. The drive for affordability requires Kaken to demonstrate clear therapeutic value and cost-effectiveness, a challenge highlighted by the government's focus on balancing innovation with cost containment as of late 2024.
| Sociological Factor | Impact on Kaken Pharmaceutical | Data/Trend (2024-2025) |
|---|---|---|
| Universal Health Coverage | Influences demand and pricing strategies; necessitates market access considerations. | Japan's system ensures widespread access, driving consistent demand. |
| Government Reimbursement Policies | Directly affects product viability and revenue; requires negotiation with health authorities. | Ministry of Health, Labour and Welfare's pricing decisions are critical; biannual revisions focus on cost control. |
| Public Perception of New Therapies | Shapes acceptance and regulatory approval speed for innovative treatments. | Public optimism for gene therapies exists, but concerns about side effects and access persist (2024 surveys). |
| Patient Advocacy | Can accelerate therapy adoption and influence market entry. | Increasing influence of patient groups in 2025 will be key for building social license. |
Technological factors
Technological advancements, particularly in artificial intelligence and genomics, are dramatically speeding up the process of finding and developing new medicines. These technologies are helping identify potential drug candidates much faster and making the development journey more efficient, ultimately aiming for quicker market entry for Kaken Pharmaceutical.
AI's impact spans the entire pharmaceutical lifecycle, from pinpointing new molecules to designing clinical trials. For instance, in 2024, numerous AI platforms are being deployed to analyze vast biological datasets, significantly reducing the time previously spent on early-stage research, which could translate to a competitive edge for Kaken.
The integration of AI in drug discovery is not just about speed; it's also about precision. By sifting through complex biological interactions, AI can predict drug efficacy and potential side effects with greater accuracy, potentially lowering development costs and increasing the success rate of clinical trials, a crucial factor for companies like Kaken.
The biotechnology sector is rapidly evolving, with advancements in genomics and gene-editing tools like CRISPR paving the way for personalized medicine. This shift towards tailored treatments, including biologics and biosimilars, promises significantly improved patient outcomes. For Kaken Pharmaceutical, these technological factors present a substantial opportunity to innovate in its core areas of dermatology, orthopedics, and infectious diseases.
The market for biologics is projected to continue its strong growth, with global sales reaching an estimated $600 billion by 2025, reflecting increasing demand for targeted therapies. Kaken's strategic investment in research and development focused on these cutting-edge biotechnologies can lead to the creation of highly specific and effective treatments, potentially capturing a larger share of this expanding market.
The increasing integration of digital health solutions and telemedicine is a significant technological factor for Kaken Pharmaceutical. Wearable diagnostics and remote patient monitoring are becoming more sophisticated, offering the potential to enhance patient care and improve drug adherence. For instance, the global telemedicine market was valued at approximately $175.7 billion in 2023 and is projected to grow substantially, indicating a strong trend towards remote healthcare delivery.
These advancements in digital health can directly benefit Kaken by providing real-time data for optimizing treatment plans and improving drug safety monitoring. Furthermore, the streamlining of clinical trials through digital platforms can accelerate the drug development process, a crucial aspect for a pharmaceutical company. By 2025, it's anticipated that many more patients will be comfortable with and actively using telehealth services, creating new avenues for Kaken's products and data collection.
Manufacturing Process Innovations
Technological advancements in pharmaceutical manufacturing are fundamentally reshaping how drugs are produced. Innovations like advanced automation and the adoption of continuous manufacturing processes are key drivers. These shifts promise greater efficiency, leading to reduced production costs and a significant uplift in product quality and consistency. For instance, continuous manufacturing can reduce facility footprint by up to 50% and cut energy consumption by 15-20% compared to traditional batch methods.
These streamlined techniques are not just about incremental improvements; they are crucial for companies like Kaken Pharmaceutical to stay competitive in a dynamic global market. The ability to quickly scale production or adapt to new product demands is paramount. By embracing these manufacturing process innovations, Kaken can ensure a more reliable supply chain and a stronger market position, directly impacting its ability to meet patient needs and shareholder expectations through 2025 and beyond.
Key manufacturing process innovations impacting the pharmaceutical sector include:
- Increased Automation: Utilizing robotics and AI for precise and repeatable tasks, reducing human error.
- Continuous Manufacturing: Shifting from batch production to integrated, end-to-end processes for improved efficiency and quality control.
- Advanced Process Analytical Technology (PAT): Real-time monitoring and control of manufacturing parameters to ensure product quality.
- Digitalization and Data Analytics: Leveraging data to optimize processes, predict maintenance needs, and improve overall operational performance.
Data Analytics and Real-Time Monitoring
Big data analytics is becoming indispensable in the pharmaceutical sector, transforming how companies like Kaken Pharmaceutical approach clinical trials and post-market surveillance. By processing vast datasets, these tools enhance decision-making across the drug lifecycle, from initial research to market strategy. For instance, in 2024, the global market for healthcare analytics was projected to reach over $50 billion, underscoring the significant investment and reliance on these technologies.
The ability to analyze big data in clinical trials allows for more precise patient selection and outcome prediction, potentially reducing trial duration and costs. This is crucial for accelerating the development of new therapies. Furthermore, real-time monitoring of drug safety through advanced analytics can quickly identify adverse events, enabling faster regulatory action and protecting patient well-being.
Kaken Pharmaceutical can leverage these technological advancements to gain deeper market insights, understanding patient needs and competitor activities more effectively. This data-driven approach is vital for optimizing R&D pipelines and commercial strategies in an increasingly competitive landscape.
- Enhanced Clinical Trial Efficiency: Big data analytics can streamline patient recruitment and data analysis, potentially shortening trial timelines.
- Improved Drug Safety Monitoring: Real-time data allows for quicker identification and response to adverse drug reactions.
- Deeper Market Insights: Analyzing market data helps in understanding patient populations and competitive landscapes more thoroughly.
- Accelerated Drug Development: The insights derived from data analytics can speed up the overall process from discovery to market launch.
Technological advancements, particularly in AI and genomics, are accelerating drug discovery and development for Kaken Pharmaceutical. AI is being deployed to analyze vast biological datasets, speeding up early-stage research and improving drug efficacy prediction. The biotech sector's evolution, with tools like CRISPR, is enabling personalized medicine, a significant opportunity for Kaken.
Digital health solutions, including telemedicine and wearables, are enhancing patient care and drug adherence. The global telemedicine market, valued at approximately $175.7 billion in 2023, demonstrates this trend. Kaken can leverage real-time data from these solutions to optimize treatments and improve drug safety monitoring.
Manufacturing innovations like increased automation and continuous manufacturing offer greater efficiency and quality. Continuous manufacturing, for instance, can reduce facility footprint by up to 50%. These process improvements are vital for Kaken to maintain competitiveness and ensure a reliable supply chain.
Big data analytics is transforming clinical trials and post-market surveillance, with the healthcare analytics market projected to exceed $50 billion in 2024. This data-driven approach allows Kaken to enhance decision-making, optimize R&D pipelines, and gain deeper market insights, ultimately accelerating drug development and improving patient outcomes.
| Technology Area | Key Advancements | Impact on Kaken Pharmaceutical | Market Data/Projections (2024-2025) |
| AI & Genomics | Accelerated drug discovery, personalized medicine | Faster R&D, improved efficacy prediction, competitive edge | Genomics market growth estimated at $30 billion by 2025 |
| Digital Health | Telemedicine, wearables, remote monitoring | Enhanced patient care, improved drug adherence, real-time data | Telemedicine market valued at $175.7 billion in 2023; projected substantial growth |
| Manufacturing Processes | Automation, continuous manufacturing, PAT | Increased efficiency, reduced costs, improved quality, supply chain reliability | Continuous manufacturing can reduce energy consumption by 15-20% |
| Big Data Analytics | Clinical trial optimization, market insights | Streamlined trials, faster development, better patient selection, improved safety monitoring | Healthcare analytics market projected to exceed $50 billion in 2024 |
Legal factors
Kaken Pharmaceutical's reliance on robust intellectual property (IP) protection, particularly patents, is paramount for safeguarding its significant research and development expenditures. This legal framework is essential for maintaining market exclusivity on its innovative pharmaceutical products, allowing for a return on investment.
The Japanese legal landscape surrounding IP, including recent litigation, underscores the intricate nature of patent term extensions and the enforcement against infringement. Navigating these complexities is crucial for Kaken's continued competitive advantage.
In 2024, the global pharmaceutical industry continued to face ongoing patent challenges, with disputes often revolving around the validity and scope of existing patents, as well as the potential for biosimilar competition. For instance, the United States Patent and Trademark Office (USPTO) reported a steady volume of patent applications and litigation related to novel drug compounds and their manufacturing processes.
Kaken's strategic approach to IP management must consider these evolving legal dynamics. The ability to secure and defend patents directly impacts the company's ability to generate revenue from its pipeline and marketed drugs, influencing future investment in R&D.
Kaken Pharmaceutical must navigate stringent drug safety and pharmacovigilance regulations, which are critical for maintaining product integrity and patient well-being. These rules mandate rigorous monitoring of adverse events and thorough reporting mechanisms across all its operational territories.
Non-compliance can lead to severe consequences, including expensive product recalls and substantial legal penalties, underscoring the need for meticulous adherence. For instance, in 2023, the global pharmaceutical industry faced significant recall costs, with certain markets seeing millions of dollars lost due to safety breaches.
The evolving landscape of regulatory oversight, particularly concerning post-market surveillance, demands continuous investment in robust pharmacovigilance systems. This is crucial as authorities like the FDA and EMA increasingly scrutinize real-world drug performance data.
Kaken Pharmaceutical must diligently adhere to antitrust and competition laws to prevent monopolistic practices and ensure a level playing field within the pharmaceutical sector. These regulations are crucial in shaping the company's pricing strategies, particularly for its key products. For instance, in 2024, the Japan Fair Trade Commission (JFTC) continued its scrutiny of pharmaceutical pricing, with a focus on ensuring fair access to medicines.
Furthermore, these laws significantly influence Kaken's potential mergers and acquisitions, acting as a gatekeeper for market entry or expansion initiatives. Any proposed consolidation or collaboration must pass regulatory review to ensure it does not unduly restrict competition. The global pharmaceutical landscape in 2024 saw increased regulatory oversight on M&A activities, with authorities in major markets like the US and EU examining deals more closely to safeguard consumer interests.
Data Privacy Regulations
Kaken Pharmaceutical must navigate a complex web of data privacy regulations, especially concerning patient data. Compliance with global standards like the General Data Protection Regulation (GDPR) and Japan's Act on the Protection of Personal Information (APPI) is paramount. These laws dictate how sensitive health information is collected, stored, processed, and shared across clinical trials, research initiatives, and commercial activities. Failure to comply can result in substantial fines and reputational damage.
The secure management of this sensitive health information is a significant legal consideration. For instance, breaches of patient data can lead to severe penalties under laws similar to HIPAA in the United States. In 2023, the total fines issued under GDPR alone exceeded €250 million, highlighting the financial risks associated with non-compliance. Kaken's commitment to robust data security protocols is therefore not just good practice but a legal imperative.
- GDPR Fines: In 2023, GDPR fines surpassed €250 million globally, underscoring the financial penalties for data privacy violations.
- Japanese APPI: Japan's Act on the Protection of Personal Information sets stringent rules for handling personal data, including health information.
- Data Security: Legal frameworks mandate secure storage and processing of sensitive patient data to prevent breaches and unauthorized access.
- Cross-border Data Transfer: Regulations often govern the international transfer of personal data, adding complexity to global operations.
Product Liability Laws
Kaken Pharmaceutical, like all drug manufacturers, operates under stringent product liability laws. These laws hold companies accountable for any adverse effects or harm a product might cause to consumers. This means Kaken must ensure its products are safe and effective, facing potential lawsuits if they fail to meet these standards.
To navigate these legal waters, rigorous quality control throughout the manufacturing process is paramount. Furthermore, comprehensive clinical trials are essential to demonstrate a drug's safety and efficacy before it reaches the market. Clear and accurate labeling, detailing potential side effects and usage instructions, is also a critical component in mitigating legal risks.
- Product Liability Claims: In 2023, the US pharmaceutical industry saw a significant number of product liability claims, with settlements and judgments often reaching millions of dollars, underscoring the financial stakes involved.
- Regulatory Scrutiny: Agencies like the FDA (Food and Drug Administration) in the US and its international counterparts impose strict guidelines on drug development and marketing, directly impacting liability exposure.
- Global Variations: Product liability laws vary considerably by country, requiring Kaken to understand and comply with the specific legal frameworks in each market where its products are sold.
Kaken Pharmaceutical's operations are heavily influenced by intellectual property laws, particularly patents, which are vital for protecting its R&D investments and ensuring market exclusivity. Navigating patent disputes and extensions in key markets like Japan remains a critical legal challenge for maintaining its competitive edge.
The company must also adhere to strict drug safety and pharmacovigilance regulations, with non-compliance leading to significant financial penalties and product recalls. Continuous investment in robust systems is necessary as regulatory bodies like the FDA and EMA increase their scrutiny of real-world drug performance data.
Antitrust and competition laws shape Kaken's pricing strategies and M&A activities, as seen in 2024 with increased regulatory oversight on deals in major markets. Compliance ensures fair market practices and prevents monopolistic tendencies, with the Japan Fair Trade Commission actively monitoring pharmaceutical pricing.
Furthermore, Kaken faces stringent data privacy regulations, such as GDPR and Japan's APPI, for handling sensitive patient data. Failure to comply, as evidenced by over €250 million in GDPR fines globally in 2023, necessitates robust data security protocols and careful consideration of cross-border data transfers.
Environmental factors
Kaken Pharmaceutical faces growing demands for sustainable manufacturing. This includes minimizing waste, lowering energy use, and conserving water in its production processes. For instance, the global pharmaceutical industry, aiming for greener operations, saw a significant push towards reducing single-use plastics in packaging, with many companies setting targets for 2025 and beyond.
To align with environmental regulations and enhance its corporate image, Kaken must integrate eco-friendly approaches. This involves optimizing its supply chain for reduced carbon emissions and adopting cleaner production technologies. The company's commitment to these practices is crucial for meeting evolving environmental standards and stakeholder expectations for social responsibility.
Kaken Pharmaceutical, like many in the industry, faces rigorous environmental impact assessments for new R&D and manufacturing sites. These assessments are crucial for ensuring adherence to stringent local and global environmental standards. For instance, in 2024, European Union regulations like the Industrial Emissions Directive continued to enforce strict controls on emissions and waste management for pharmaceutical manufacturing facilities.
The management of emissions, wastewater discharge, and hazardous waste is a significant aspect of these assessments. Pharmaceutical production processes can generate complex waste streams requiring specialized treatment. In 2025, the focus on sustainable manufacturing practices is expected to intensify, with increased scrutiny on water usage and the reduction of pharmaceutical residues in wastewater, potentially impacting operational costs for facilities like Kaken's.
Climate change is increasingly altering disease patterns, a significant factor for Kaken Pharmaceutical. Rising global temperatures and changing precipitation can expand the geographical reach of vector-borne illnesses like dengue fever and Lyme disease. For instance, the World Health Organization (WHO) reported in 2024 that the incidence of dengue fever has surged by 30 times globally over the last two decades, directly linked to warmer climates. This shift may necessitate Kaken to re-evaluate its research and development priorities, potentially increasing investment in treatments for these emerging infectious diseases and influencing market demand for its existing products.
Compliance with Environmental Regulations
Kaken Pharmaceutical must navigate a complex web of environmental regulations across its global operations. This includes strict adherence to standards concerning greenhouse gas (GHG) emissions, the responsible disposal of chemical waste, and comprehensive pollution control measures. Failure to comply can result in significant penalties and reputational damage.
Japan's own healthcare policy is increasingly emphasizing environmental sustainability. A key aspect of this is the push to reduce GHG emissions within the medical sector. For instance, the Japanese government has set targets for emission reductions across various industries, and the pharmaceutical sector is expected to contribute to these national goals. This means Kaken will likely face heightened scrutiny and expectations regarding its environmental footprint.
- Japan's commitment to carbon neutrality by 2050 necessitates stricter environmental controls across all industries, including pharmaceuticals.
- Pharmaceutical manufacturing often involves complex chemical processes that require careful waste management to prevent environmental contamination.
- Global investors and consumers are increasingly prioritizing companies with strong environmental, social, and governance (ESG) performance, impacting Kaken's access to capital and market share.
Corporate Social Responsibility (CSR) and Environmental Stewardship
Kaken Pharmaceutical, like many in the industry, faces increasing pressure from investors and the public to show strong Corporate Social Responsibility (CSR) and environmental stewardship. This means not just talking about environmental protection but actively demonstrating it through transparent reporting and embedding sustainability into their core business strategy. For instance, as of early 2024, there's a noticeable trend where companies with robust ESG (Environmental, Social, and Governance) practices are seeing higher valuations, with some studies indicating a premium of up to 18% for top-tier ESG performers.
The pharmaceutical sector, in particular, is scrutinized for its environmental footprint, from manufacturing processes to waste disposal. Kaken's commitment to sustainability could translate into tangible actions like reducing greenhouse gas emissions from its operations. Data from 2023 shows that leading pharmaceutical companies have set ambitious targets, with many aiming for carbon neutrality by 2030 or 2040, often backed by significant investments in renewable energy and circular economy principles.
- Investor Scrutiny: Stakeholders are increasingly prioritizing companies with clear environmental policies and performance metrics, impacting capital allocation decisions.
- Regulatory Trends: Evolving environmental regulations worldwide compel businesses to adopt more sustainable practices, often leading to innovation and efficiency gains.
- Supply Chain Impact: Kaken's environmental performance is also judged by its supply chain's practices, pushing for greater accountability across all operational levels.
- Public Perception: A strong environmental record enhances brand reputation and customer loyalty, which is crucial in the healthcare sector.
Kaken Pharmaceutical must address the growing demand for sustainable operations, including waste reduction and energy efficiency, as seen in the pharmaceutical industry's 2025 targets for reducing single-use plastics. Aligning with environmental regulations and enhancing its image necessitates eco-friendly approaches, such as optimizing supply chains for lower carbon emissions, a trend reinforced by EU directives like the Industrial Emissions Directive in 2024.
The company faces rigorous environmental impact assessments for new facilities, ensuring compliance with global standards, and must manage emissions and waste streams, with intensified scrutiny on water usage and pharmaceutical residues expected by 2025. Furthermore, climate change is altering disease patterns, potentially shifting Kaken's R&D focus towards emerging infectious diseases, a trend highlighted by the WHO's 2024 report on the 30-fold increase in dengue fever incidence linked to warmer climates.
| Environmental Factor | Impact on Kaken Pharmaceutical | 2024/2025 Data/Trends |
|---|---|---|
| Sustainability Demands | Pressure for greener manufacturing, waste reduction, and energy efficiency. | Industry-wide push to reduce single-use plastics by 2025; increased focus on ESG performance. |
| Regulatory Compliance | Adherence to strict environmental laws regarding emissions, waste, and pollution. | EU Industrial Emissions Directive continues to enforce controls; Japan's carbon neutrality goals impact sector. |
| Climate Change Impact | Potential shifts in R&D priorities due to changing disease patterns. | 30x increase in global dengue fever incidence since 2000 linked to climate change (WHO, 2024). |
| Investor & Public Perception | Growing importance of CSR and ESG performance for capital access and brand reputation. | Companies with strong ESG practices saw higher valuations (up to 18% premium) in early 2024; leading pharma aiming for carbon neutrality by 2030/2040. |
PESTLE Analysis Data Sources
Our PESTLE Analysis for Kaken Pharmaceutical is grounded in data from official government health agencies, international pharmaceutical regulatory bodies, and reputable market research firms. This ensures a comprehensive understanding of the political, economic, social, technological, legal, and environmental factors influencing Kaken's operations.
