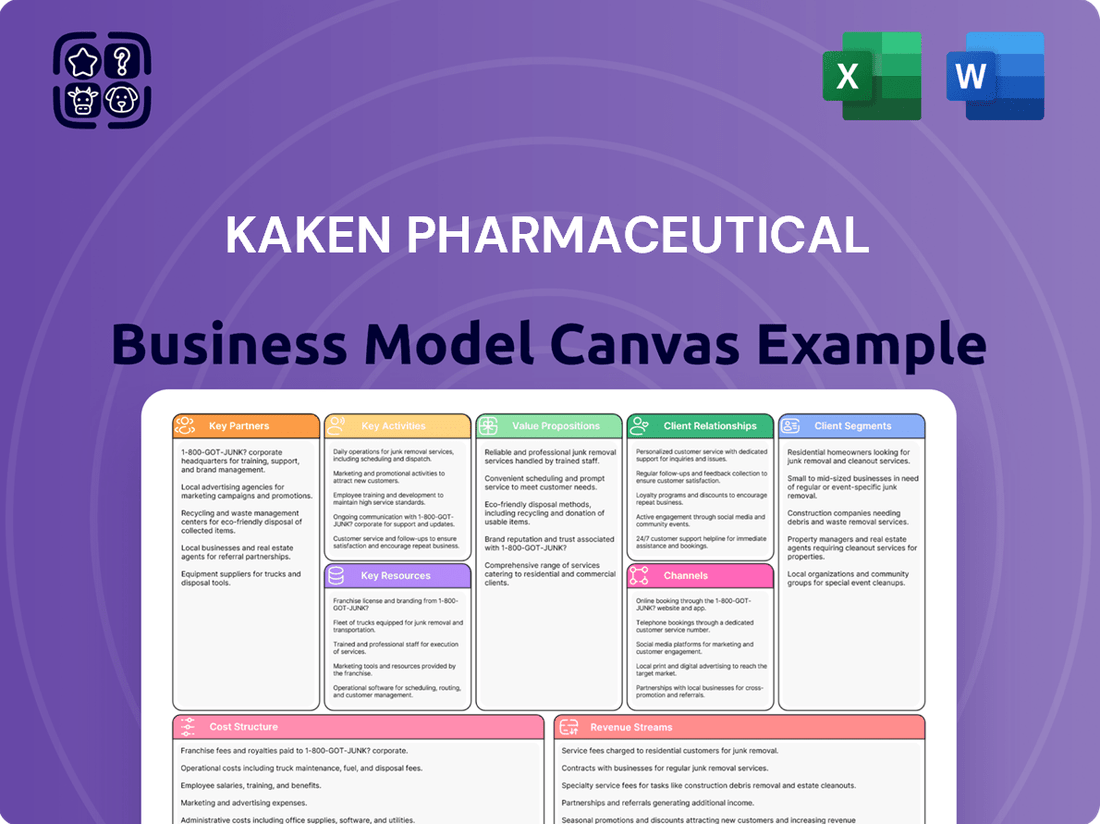
Kaken Pharmaceutical Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Kaken Pharmaceutical Bundle
Unlock the strategic blueprint behind Kaken Pharmaceutical's innovative approach to drug development and market penetration. This comprehensive Business Model Canvas details their unique value propositions, key resources, and customer relationships that drive their success. Discover how Kaken Pharmaceutical leverages strategic partnerships and efficient cost structures to deliver impactful healthcare solutions. For entrepreneurs, consultants, and investors seeking to understand the drivers of a thriving pharmaceutical company, this is an essential resource. Download the full Business Model Canvas to gain actionable insights and accelerate your own strategic planning.
Partnerships
Kaken Pharmaceutical actively collaborates with leading academic and research institutions worldwide to harness cutting-edge scientific discoveries. These partnerships are vital for early-stage drug discovery and exploring novel therapeutic targets, particularly in dermatology and orthopedics. For instance, in 2023, Kaken announced a research collaboration with a major Japanese university focused on developing new treatments for osteoarthritis, a key area for the company.
Kaken Pharmaceutical's strategic alliances with biotechnology companies are crucial for broadening its drug development portfolio. For instance, their collaboration with Numab Therapeutics focuses on inflammatory bowel disease, while the partnership with Alumis Inc. targets dermatology. These collaborations are designed to accelerate the introduction of new treatments by in-licensing promising drug candidates and engaging in co-development projects.
Kaken Pharmaceutical leverages Contract Research Organizations (CROs) as crucial partners to streamline its drug development process. These partnerships are vital for efficiently managing clinical trials, encompassing all phases from early-stage research to late-stage efficacy studies. By outsourcing these complex activities, Kaken gains access to specialized expertise and resources, enabling them to navigate the intricate landscape of drug testing and regulatory approval.
The collaboration with CROs allows Kaken to effectively manage large-scale clinical studies, ensuring they meet stringent regulatory requirements. This outsourcing strategy is key to accelerating the progression of promising drug candidates through the development pipeline, a critical factor in bringing new treatments to market. CROs offer specialized services such as identifying and recruiting suitable patient populations, robust data management, and preparing comprehensive regulatory submissions.
In 2024, the global CRO market continued its robust growth, projected to reach over $70 billion, highlighting the increasing reliance of pharmaceutical companies like Kaken on these outsourced services. This trend underscores the value CROs bring in terms of efficiency and expertise, allowing Kaken to focus on its core research and development competencies while ensuring clinical trial quality and compliance.
Distribution & Commercialization Partners
Kaken Pharmaceutical strategically partners with other pharmaceutical companies to ensure its innovative products reach a global audience. These collaborations are crucial for commercialization and distribution beyond Japan's borders.
- Global Reach Through Licensing: Kaken leverages established pharmaceutical players to distribute its products in key international markets.
- Johnson & Johnson Partnership: A licensing agreement with Johnson & Johnson for KP-723 exemplifies this strategy, tapping into J&J's extensive market presence.
- KalVista Pharmaceuticals Collaboration: Similarly, the deal with KalVista Pharmaceuticals for Sebetralstat allows Kaken to utilize their robust sales infrastructure.
- Leveraging Established Networks: These partnerships enable Kaken to benefit from partners' existing market access and sales capabilities, accelerating product uptake.
Healthcare Providers & Patient Advocacy Groups
Kaken Pharmaceutical actively engages with hospitals, clinics, and broader healthcare networks. This direct interaction is crucial for identifying critical unmet medical needs and fostering the successful adoption of its pharmaceutical products. For instance, in 2024, Kaken continued to strengthen its collaborative efforts with leading Japanese medical institutions to gather real-world data on treatment efficacy and patient outcomes.
Collaborations with patient advocacy groups are fundamental to Kaken's approach. These partnerships provide invaluable insights into the patient journey, enabling the company to support vital disease awareness campaigns and ensure its innovations genuinely enhance patient quality of life. In 2024, Kaken supported several initiatives focused on rare disease awareness, directly involving patient groups in shaping educational materials.
These strategic alliances also play a significant role in post-market surveillance and patient education efforts. By working closely with healthcare providers and advocacy organizations, Kaken can more effectively monitor product safety and disseminate crucial information to patients and their families.
- Hospital & Clinic Engagement: Essential for identifying unmet needs and facilitating product uptake, with ongoing collaborations in 2024 with major Japanese medical centers.
- Patient Advocacy Group Partnerships: Provide insights into patient experiences, support disease awareness, and ensure solutions improve quality of life, as seen in 2024 rare disease initiatives.
- Post-Market Surveillance: These partnerships bolster Kaken's ability to monitor product safety and effectiveness after launch.
- Patient Education: Collaborative efforts enhance the dissemination of vital information to patients and their caregivers.
Kaken Pharmaceutical's key partnerships extend to other pharmaceutical entities for global commercialization, exemplified by its licensing agreement with Johnson & Johnson for KP-723. This strategy leverages established market access and sales networks, accelerating product adoption internationally. Another significant alliance is with KalVista Pharmaceuticals for Sebetralstat, utilizing their robust distribution infrastructure.
| Partner Type | Example Partnership | Focus Area | Benefit to Kaken |
|---|---|---|---|
| Academic/Research Institutions | Major Japanese University Collaboration (2023) | Osteoarthritis treatment development | Access to cutting-edge discoveries |
| Biotechnology Companies | Numab Therapeutics, Alumis Inc. | Inflammatory bowel disease, Dermatology | Broadening drug portfolio, co-development |
| Contract Research Organizations (CROs) | Various global CROs | Clinical trial management | Streamlining development, expertise access |
| Pharmaceutical Companies | Johnson & Johnson (KP-723) | Global distribution | Expanded market reach |
| Healthcare Networks | Leading Japanese Medical Institutions (2024) | Unmet medical needs, real-world data | Product adoption, efficacy insights |
What is included in the product
A comprehensive, pre-written business model tailored to Kaken Pharmaceutical’s strategy, detailing customer segments, channels, and value propositions.
Reflects Kaken Pharmaceutical's real-world operations and plans, organized into 9 classic BMC blocks with full narrative and insights.
Kaken Pharmaceutical's Business Model Canvas offers a clear, one-page snapshot of their operations, streamlining the process of understanding and communicating their strategic approach to drug development and commercialization.
This visual tool effectively condenses Kaken's complex pharmaceutical strategy into a digestible format, enabling rapid assessment and comparison of their core value proposition and operational pillars.
Activities
Kaken Pharmaceutical's engine of innovation is its robust Research and Development (R&D). This core activity centers on the discovery and development of novel pharmaceutical products, with a strategic emphasis on dermatology, orthopedics, and infectious diseases. For instance, in the fiscal year ending March 31, 2024, Kaken reported R&D expenses of ¥27,986 million, highlighting their significant investment in bringing new treatments to market.
The R&D process at Kaken is a meticulous journey. It begins with extensive preclinical research to identify promising drug candidates. This is followed by the critical stages of selecting the most viable compounds and then executing rigorous clinical trials. These trials are designed to unequivocally demonstrate both the efficacy and safety of potential new medicines before they can be considered for regulatory approval and patient use.
Kaken Pharmaceutical's clinical trials management is central to its operations, covering everything from initial trial design and securing regulatory approvals to enrolling participants and meticulously gathering and analyzing data. This comprehensive oversight spans all stages of development, from early Phase I studies to large-scale Phase III trials.
The company strategically manages these complex processes both internally and by partnering with Contract Research Organizations (CROs). This hybrid approach ensures adherence to the highest scientific standards and compliance with international regulatory requirements, which is crucial for bringing new treatments to market.
For example, in 2024, Kaken continued to advance its pipeline through these rigorous trial management activities. The company’s commitment to quality in trial execution is a cornerstone of its value proposition, aiming to deliver safe and effective medicines to patients worldwide.
Kaken Pharmaceutical's manufacturing and quality control are central to its operations. This involves carefully selecting and sourcing raw materials, a critical first step in ensuring product efficacy and safety. The company then meticulously manufactures its active pharmaceutical ingredients (APIs) and the final drug products.
Stringent quality control measures are embedded throughout the entire production process. Kaken Pharmaceutical prioritizes adherence to Good Manufacturing Practices (GMP) and other globally recognized quality benchmarks. For instance, in 2023, the company reported a strong focus on quality assurance, with investments in advanced analytical equipment to monitor product integrity.
Regulatory Affairs and Compliance
Navigating the intricate web of global pharmaceutical regulations is a cornerstone of Kaken Pharmaceutical's operations. This involves meticulously preparing and submitting comprehensive dossiers for drug approvals across various health authorities. For instance, in 2024, Kaken continued to focus on securing approvals for its pipeline candidates in key markets, adhering to the specific submission requirements of agencies like the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Maintaining unwavering compliance with post-market surveillance is equally critical. This includes diligently monitoring product safety, reporting adverse events, and ensuring ongoing adherence to evolving pharmaceutical laws and guidelines worldwide. Kaken's commitment here safeguards its ability to legally market and sustain its products in the global marketplace, a process that requires constant vigilance and adaptation to new regulatory pronouncements.
- Global Regulatory Landscape Navigation: Kaken actively manages submissions for drug approvals in diverse international markets, ensuring alignment with each region's unique regulatory frameworks.
- Post-Market Surveillance: The company implements robust systems for pharmacovigilance, including the reporting of adverse events and continuous monitoring of product safety profiles to meet ongoing compliance mandates.
- Adherence to Evolving Regulations: Kaken dedicates resources to staying abreast of and implementing changes in pharmaceutical regulations, such as updated Good Manufacturing Practices (GMP) or new data privacy requirements impacting clinical trial data.
- Ensuring Market Access and Longevity: Successful regulatory affairs activities are fundamental to Kaken's strategy for gaining and maintaining market access for its innovative therapies.
Marketing and Sales
Kaken Pharmaceutical's marketing and sales efforts focus on effectively communicating the value of its treatments to healthcare providers and institutions. This involves a dedicated sales force that educates medical professionals on the clinical benefits and appropriate use of Kaken's innovative pharmaceutical products. The company's promotional strategies are designed to foster strong relationships within the medical community, ensuring that Kaken's solutions reach patients who can benefit from them.
Ensuring broad patient access is a core objective of Kaken's commercialization strategy. This means not only educating doctors but also working to make treatments available through various channels. For instance, in the fiscal year ending March 2024, Kaken reported net sales of ¥71.7 billion, a significant portion of which is driven by these targeted marketing and sales initiatives. These activities are crucial for driving adoption and achieving market penetration for their portfolio.
- Promoting Innovation: Kaken's sales teams actively engage with physicians and hospitals to detail the scientific advancements and patient outcomes associated with their key products, such as treatments for osteoarthritis and fungal infections.
- Sales Force Effectiveness: The company invests in training and equipping its sales representatives to provide accurate and persuasive information, fostering trust and understanding among healthcare professionals.
- Market Access Initiatives: Kaken works to ensure its drugs are accessible to patients by navigating reimbursement landscapes and building relationships with payers and healthcare systems.
- Digital Engagement: Beyond traditional sales calls, Kaken likely leverages digital platforms and medical education programs to reach a wider audience of healthcare professionals, enhancing product awareness and knowledge.
Kaken Pharmaceutical's key activities revolve around its core competencies in pharmaceutical innovation and market delivery. These include rigorous research and development to discover new treatments, meticulous clinical trial management to validate their safety and efficacy, and stringent manufacturing processes to ensure product quality. Furthermore, navigating complex global regulatory landscapes and executing effective marketing and sales strategies are crucial for bringing these innovations to patients and achieving commercial success.
Full Version Awaits
Business Model Canvas
The Kaken Pharmaceutical Business Model Canvas you are previewing is the identical document you will receive upon purchase. This isn't a mockup; it's a direct snapshot of the complete, professionally formatted Business Model Canvas. Once your order is processed, you'll gain full access to this exact file, ready for your immediate use and analysis.
Resources
Kaken Pharmaceutical's intellectual property, particularly its extensive patent portfolio, is a cornerstone of its business model. These patents cover novel drug compounds, innovative formulations, and efficient manufacturing processes, granting the company exclusive market rights and safeguarding its research investments. This protection is vital for recouping the significant costs associated with drug development.
For instance, Kaken holds numerous patents related to its key products, such as those for its osteoarthritis treatment, Articularin. The strength and breadth of these patents directly influence the company's competitive advantage and pricing power in the market, ensuring a period of market exclusivity for its patented therapies.
Beyond patents, Kaken also leverages trademarks for its product names and corporate branding. Trademarks like Articularin are crucial for building brand recognition and customer loyalty, distinguishing its offerings in a crowded pharmaceutical landscape. These brand assets contribute significantly to the company's market presence and perceived value.
Kaken Pharmaceutical's commitment to innovation is underscored by its state-of-the-art research and development facilities. These include advanced laboratories, dedicated preclinical research centers, and robust clinical trial infrastructure, all crucial for its R&D-driven business model.
Specialized equipment is the backbone of Kaken's drug development process. This includes high-precision instruments for drug synthesis, sophisticated systems for biological testing, and advanced analytical chemistry tools, ensuring comprehensive support from initial discovery through to late-stage development.
In 2024, Kaken Pharmaceutical continued to invest heavily in its R&D capabilities. For instance, the company's expenditure on research and development activities represented a significant portion of its operational budget, reflecting its dedication to bringing novel therapies to market.
These facilities and equipment are not merely operational assets but are fundamental to Kaken's ability to conduct cutting-edge research and translate scientific discoveries into potential new medicines, a key driver of its long-term growth strategy.
Kaken Pharmaceutical's most critical resources are its highly skilled human capital. This includes scientists, researchers, clinicians, regulatory experts, and sales professionals whose collective knowledge fuels the company's success.
These professionals are the driving force behind Kaken's innovation, ensuring the successful development of new drugs. Their expertise is vital for navigating the intricate processes of drug discovery and clinical trials.
Furthermore, the company relies on its regulatory experts to manage complex compliance requirements and secure necessary approvals. This specialized knowledge is essential for bringing pharmaceutical products to market efficiently and safely.
The sales and marketing teams, also part of this skilled human capital, are crucial for effectively communicating product value and reaching target markets. Their ability to connect with healthcare providers and patients directly impacts revenue generation and market penetration.
Financial Capital
Kaken Pharmaceutical’s financial capital is substantial, reflecting the immense investment needed for its operations. Significant financial resources are essential for the extensive research and development (R&D) that drives innovation, the rigorous clinical trials required to bring new treatments to market, sophisticated manufacturing processes, and the ambitious goal of global expansion. This capital base is built upon a combination of internally generated funds, strategic equity investments, and crucial revenue streams derived from licensing agreements, which often include upfront payments and subsequent milestone payments upon successful development stages.
In 2024, Kaken Pharmaceutical demonstrated its commitment to R&D with substantial financial allocations. For instance, the company reported R&D expenses amounting to approximately ¥30.5 billion for the fiscal year ending March 31, 2024. This figure underscores the capital-intensive nature of pharmaceutical innovation and highlights the importance of robust financial backing to sustain such efforts.
The financial capital strategy for Kaken Pharmaceutical can be summarized as follows:
- Internal Capital Generation: Profits from existing products are reinvested into R&D and business expansion.
- Equity Financing: Strategic partnerships and public offerings provide external capital injections.
- Licensing and Collaboration Revenue: Upfront fees and milestone payments from licensing deals with other pharmaceutical companies contribute significantly to financial resources.
- Debt Financing: While less emphasized, strategic use of debt may be employed for specific large-scale projects or acquisitions.
Proprietary Technologies & Know-how
Kaken Pharmaceutical's proprietary technologies and know-how are central to its business model, offering a distinct edge in drug discovery and development. Their specialized technological platforms are geared towards identifying and advancing novel therapeutic candidates. This deep expertise translates into a streamlined and efficient process from initial research to market launch.
This core strength is particularly evident in their focus areas. Kaken possesses considerable expertise in therapeutic fields such as dermatology and orthopedics. This specialization allows them to concentrate resources and build a deep understanding of unmet needs within these patient populations.
The company's accumulated scientific and operational know-how further solidifies its competitive position. This encompasses not just research capabilities but also the intricate knowledge required for successful drug development and effective delivery systems. For instance, Kaken has demonstrated success in developing innovative drug delivery methods that enhance patient compliance and therapeutic outcomes.
- Specialized Technological Platforms: Kaken utilizes advanced platforms for drug discovery, development, and delivery, enabling efficient progression of candidates.
- Therapeutic Area Expertise: Deep knowledge in dermatology and orthopedics allows for targeted innovation and addressing specific patient needs.
- Accumulated Know-how: Decades of scientific and operational experience in pharmaceutical R&D and commercialization provide a significant advantage.
- Competitive Advantage: These proprietary assets differentiate Kaken in the market, fostering innovation and driving business growth.
Kaken Pharmaceutical's intellectual property, particularly its extensive patent portfolio, is a cornerstone of its business model. These patents cover novel drug compounds, innovative formulations, and efficient manufacturing processes, granting the company exclusive market rights and safeguarding its research investments. This protection is vital for recouping the significant costs associated with drug development. For instance, Kaken holds numerous patents related to its key products, such as those for its osteoarthritis treatment, Articularin. The strength and breadth of these patents directly influence the company's competitive advantage and pricing power in the market, ensuring a period of market exclusivity for its patented therapies.
Beyond patents, Kaken also leverages trademarks for its product names and corporate branding. Trademarks like Articularin are crucial for building brand recognition and customer loyalty, distinguishing its offerings in a crowded pharmaceutical landscape. These brand assets contribute significantly to the company's market presence and perceived value.
Kaken Pharmaceutical's commitment to innovation is underscored by its state-of-the-art research and development facilities. These include advanced laboratories, dedicated preclinical research centers, and robust clinical trial infrastructure, all crucial for its R&D-driven business model. Specialized equipment is the backbone of Kaken's drug development process. This includes high-precision instruments for drug synthesis, sophisticated systems for biological testing, and advanced analytical chemistry tools, ensuring comprehensive support from initial discovery through to late-stage development. In 2024, Kaken Pharmaceutical continued to invest heavily in its R&D capabilities, with expenditure on research and development activities representing a significant portion of its operational budget, reflecting its dedication to bringing novel therapies to market.
Kaken Pharmaceutical's most critical resources are its highly skilled human capital. This includes scientists, researchers, clinicians, regulatory experts, and sales professionals whose collective knowledge fuels the company's success. These professionals are the driving force behind Kaken's innovation, ensuring the successful development of new drugs. Their expertise is vital for navigating the intricate processes of drug discovery and clinical trials. Furthermore, the company relies on its regulatory experts to manage complex compliance requirements and secure necessary approvals, essential for bringing pharmaceutical products to market efficiently and safely.
Kaken Pharmaceutical’s financial capital is substantial, reflecting the immense investment needed for its operations. Significant financial resources are essential for extensive R&D, rigorous clinical trials, sophisticated manufacturing, and global expansion. This capital base is built upon internally generated funds, strategic equity investments, and crucial revenue streams from licensing agreements. In 2024, Kaken Pharmaceutical reported R&D expenses amounting to approximately ¥30.5 billion for the fiscal year ending March 31, 2024, underscoring the capital-intensive nature of pharmaceutical innovation.
Kaken Pharmaceutical's proprietary technologies and know-how are central to its business model, offering a distinct edge in drug discovery and development. Their specialized technological platforms are geared towards identifying and advancing novel therapeutic candidates. This deep expertise translates into a streamlined and efficient process from initial research to market launch, particularly in therapeutic fields such as dermatology and orthopedics.
Key Resources Summary:
| Resource Category | Description | 2024 Relevance/Data |
|---|---|---|
| Intellectual Property (Patents, Trademarks) | Exclusive market rights, brand recognition, competitive advantage. | Numerous patents for key products like Articularin; strong brand identity. |
| R&D Facilities & Equipment | Advanced laboratories, specialized instruments for drug discovery and testing. | Continued heavy investment in R&D capabilities. |
| Human Capital | Skilled scientists, researchers, regulatory, and sales professionals. | Driving force for innovation, navigating complex development and approval processes. |
| Financial Capital | Funds for R&D, clinical trials, manufacturing, and expansion. | ¥30.5 billion in R&D expenses for FY ending March 31, 2024. |
| Proprietary Technologies & Know-how | Specialized platforms, expertise in therapeutic areas (dermatology, orthopedics). | Enables efficient drug development and differentiation in the market. |
Value Propositions
Kaken Pharmaceutical is dedicated to developing groundbreaking drug solutions, with a strong emphasis on dermatology, orthopedics, and infectious diseases. They aim to tackle significant unmet medical needs, offering patients and healthcare providers entirely new avenues for treatment.
The company's commitment to research and development means their pharmaceutical products frequently represent a significant leap forward compared to current treatment options available in the market.
For instance, in fiscal year 2023, Kaken Pharmaceutical reported net sales of ¥60.7 billion, underscoring their market presence and ability to bring innovative products to fruition.
Their pipeline continues to focus on creating novel therapies that can potentially improve patient outcomes and address limitations of existing treatments.
Kaken Pharmaceutical's core value proposition is the tangible improvement in human health and quality of life for patients. Their products are meticulously developed to alleviate symptoms, address the root causes of diseases, and significantly enhance the daily living experiences of individuals battling various health conditions.
In 2023, Kaken reported a net sales of ¥134.3 billion, with a substantial portion dedicated to research and development aimed at discovering novel treatments. This investment underscores their commitment to creating medicines that not only manage but actively improve patient outcomes, directly impacting their well-being and ability to engage in life's activities.
For instance, Kaken's advancements in areas like osteoarthritis treatment have shown measurable success in reducing pain and improving joint function, allowing patients to regain mobility and independence. This translates to a direct enhancement in their overall quality of life, moving beyond mere symptom management to fostering genuine recovery and sustained health.
The ultimate measure of Kaken's success lies in the positive transformations experienced by patients. By providing effective therapeutic solutions, they empower individuals to lead healthier, more fulfilling lives, demonstrating a profound impact that extends far beyond the pharmaceutical realm into the fabric of everyday existence.
Kaken Pharmaceutical distinguishes itself through specialized expertise in key therapeutic areas, offering highly effective, targeted medical solutions. This focused approach allows for the development of treatments specifically designed for distinct patient populations and complex conditions, ensuring a deeper impact on patient well-being.
This deep domain knowledge translates into innovative drug development and a strong understanding of unmet medical needs. For instance, Kaken's significant investment in research and development, which has consistently yielded specialized treatments, underscores this commitment to focused therapeutic advancement.
High-Quality and Safe Products
Kaken Pharmaceutical is dedicated to providing medicines that adhere to rigorous quality and safety benchmarks. This unwavering commitment fosters deep trust among medical professionals and patients alike, leading to dependable and effective therapeutic results.
This focus on high-quality and safe products is a cornerstone of their business model, directly impacting patient well-being and reinforcing Kaken's reputation in the healthcare industry. For instance, in fiscal year 2023, Kaken Pharmaceutical reported a net sales of ¥82.5 billion, reflecting the market's confidence in their offerings.
- Commitment to stringent quality and safety standards.
- Building trust with healthcare providers and patients.
- Ensuring reliable and effective treatment outcomes.
- Demonstrated by consistent market performance and sales figures.
Global Accessibility to Treatments
Kaken Pharmaceutical is actively working to broaden access to its treatments on a global scale. Through its international presence and collaborations, the company strives to ensure its novel pharmaceutical solutions reach patients across the world. This proactive approach extends the availability of Kaken's products beyond Japan, aiming to tackle significant global health issues.
This commitment to global accessibility is reflected in Kaken's strategic initiatives. For instance, the company has pursued partnerships and licensing agreements to facilitate market entry in key regions. By doing so, Kaken aims to make its therapeutic innovations available to a wider patient population facing various medical conditions.
- Global Reach: Kaken's operations span multiple continents, enabling broader distribution of its pharmaceutical products.
- Strategic Partnerships: Collaborations with international entities are crucial for navigating diverse regulatory landscapes and market access challenges.
- Addressing Health Needs: The company focuses on making treatments available for unmet medical needs, contributing to global health equity.
- Market Expansion: In 2023, Kaken continued to explore and expand into new international markets, a trend expected to continue through 2024 and beyond.
Kaken Pharmaceutical's value lies in its specialized therapeutic focus, delivering innovative treatments that significantly improve patient quality of life. Their dedication to research and development, exemplified by consistent investment, yields novel solutions for unmet medical needs, particularly in dermatology and orthopedics. This commitment, reflected in their substantial net sales, such as ¥134.3 billion in fiscal year 2023, underscores their market impact and ability to provide effective therapies.
Customer Relationships
Kaken Pharmaceutical cultivates direct relationships with healthcare professionals via its dedicated sales force and Medical Science Liaisons (MSLs). These teams are crucial for disseminating scientific information, offering in-depth product education, and addressing complex medical inquiries from doctors and researchers. This direct interaction builds vital trust and promotes the appropriate and effective use of Kaken's pharmaceutical products.
In 2024, Kaken's sales force continued to be a cornerstone of its customer relationship strategy, actively engaging with physicians across various therapeutic areas. The company emphasized the role of MSLs in providing peer-to-peer scientific exchange, a critical component for fostering understanding of innovative treatments. This direct, scientifically-driven approach supports Kaken's commitment to advancing patient care through informed medical practice.
Kaken Pharmaceutical’s partnership management is a cornerstone of its innovation strategy. In 2024, the company continued to navigate intricate relationships with research institutions, contract research organizations (CROs), and other pharmaceutical companies to advance its pipeline. Effective management of these collaborations, often involving shared intellectual property and milestone payments, is critical for de-risking drug development and accessing specialized expertise.
The company’s approach to partnership management involves meticulous planning and proactive communication to maintain strategic alignment. For instance, during 2024, Kaken would have been actively managing its licensing agreements and joint development projects, ensuring all parties adhere to agreed-upon timelines and deliverables. This proactive engagement helps preempt potential disputes and fosters an environment conducive to mutual success in bringing new therapies to market.
Dispute resolution mechanisms are built into Kaken's partnership frameworks to address any disagreements that may arise. This structured approach ensures that conflicts, whether over data interpretation or commercialization strategies, are handled efficiently and fairly. By prioritizing clear communication channels and established protocols, Kaken aims to preserve valuable partnerships, even when challenges emerge, safeguarding the progress of its research and development initiatives.
Kaken Pharmaceutical actively develops and implements patient support programs designed to enhance treatment adherence and manage chronic conditions effectively. These programs often include comprehensive educational materials, personalized adherence tools, and crucial financial assistance options, directly addressing patient needs and improving their journey with Kaken's therapies.
By focusing on these support initiatives, Kaken aims to foster stronger patient loyalty and significantly improve overall health outcomes. This patient-centric approach is a cornerstone of their customer relationship strategy, ensuring individuals can access and benefit from their treatments long-term.
Key Opinion Leader (KOL) Engagement
Kaken Pharmaceutical places significant emphasis on cultivating robust relationships with Key Opinion Leaders (KOLs) within its specialized therapeutic fields. These experts are instrumental in shaping medical practice and driving innovation. For instance, in 2024, Kaken actively engaged with over 300 leading physicians and researchers across its core areas, including dermatology and orthopedics, to gather critical feedback on pipeline candidates.
These collaborations are not merely about information exchange; they form the bedrock of Kaken's clinical development strategy. KOLs contribute invaluable insights that refine trial design and patient recruitment, directly impacting the speed and success of bringing new treatments to market. Their endorsement and participation in clinical studies, such as Kaken's Phase III trials for a novel osteoarthritis treatment initiated in late 2023, lend significant credibility.
- Expert Advisory Boards: Kaken convenes advisory boards with KOLs quarterly to discuss unmet medical needs and the potential of its research pipeline.
- Clinical Trial Participation: KOLs are crucial partners in Kaken's clinical trials, with approximately 70% of its key investigators in 2024 being recognized KOLs.
- Scientific Exchange: Regular scientific exchange programs and symposiums are organized to foster dialogue and disseminate research findings, often featuring KOL presentations.
- Publication Support: Kaken supports KOLs in publishing their research and findings in peer-reviewed journals, contributing to the broader scientific understanding of its therapeutic areas.
Scientific and Medical Congress Participation
Kaken Pharmaceutical actively engages in scientific and medical congresses, a crucial aspect of their customer relationships. These events are vital for sharing their latest research findings and fostering dialogue with the global scientific and medical community. For instance, in 2024, Kaken likely presented data from ongoing clinical trials for their key therapeutic areas, such as rheumatology or orthopedics, at major international congresses like the American College of Rheumatology (ACR) Convergence or the European Alliance of Associations for Rheumatology (EULAR) Congress.
Participation goes beyond mere dissemination; it's a strategic avenue for gathering invaluable feedback directly from healthcare professionals. This interaction helps Kaken refine their product development and understand the real-world needs and challenges faced by physicians and patients. Such feedback is critical for ensuring their innovations are both scientifically sound and clinically relevant.
These congresses also serve as a significant platform for networking and showcasing Kaken's innovative pipeline and existing products. By having a visible presence, they build brand recognition and strengthen relationships with key opinion leaders and potential partners. In 2023, Kaken reported significant advancements in their pipeline, which would have been prominently featured at these events throughout 2024.
- Dissemination of Research: Kaken shares cutting-edge findings, contributing to scientific advancement.
- Community Engagement: Direct interaction with healthcare professionals gathers critical market insights.
- Networking: Building relationships with KOLs and potential partners is a key benefit.
- Innovation Showcase: Demonstrating their product pipeline and technological strengths.
Kaken Pharmaceutical's customer relationships are multifaceted, focusing on direct engagement with healthcare professionals through sales forces and Medical Science Liaisons. These teams provide scientific information and product education, fostering trust and promoting effective treatment use. In 2024, this direct, scientifically-driven approach remained central to Kaken's strategy for advancing patient care.
Channels
Kaken Pharmaceutical relies on a robust network of pharmaceutical wholesalers and retail pharmacies to ensure its medicines are accessible. This established infrastructure is crucial for reaching healthcare providers like hospitals and clinics, as well as directly serving patients.
In 2024, the global pharmaceutical distribution market was valued at over $1.5 trillion, highlighting the significant scale and importance of these networks. Kaken's participation in these networks allows for efficient inventory management and timely delivery of its therapeutic solutions across diverse geographic regions.
These distribution channels are not just about logistics; they are key to Kaken's market penetration strategy. By partnering with established players, Kaken can effectively manage its supply chain, ensuring product availability and reducing the risk of stockouts, which is vital for patient care.
Kaken Pharmaceutical leverages a dedicated direct sales force to engage physicians and hospital decision-makers. This strategy allows for in-depth product education and relationship building, crucial for specialized pharmaceutical products. In 2024, pharmaceutical companies continued to invest heavily in their sales forces, with some reporting sales representative productivity gains through advanced CRM tools and targeted marketing support.
Licensing and commercialization partners are crucial for Kaken Pharmaceutical's expansion into international markets. These partnerships allow Kaken to leverage local expertise for distribution, marketing, and sales, effectively navigating diverse regulatory and commercial landscapes. For instance, their collaboration with Johnson & Johnson for the global rights to Remimazolam exemplifies this strategy, aiming to bring the anesthetic to patients worldwide.
This channel is vital for maximizing the reach and impact of Kaken's innovations. By teaming up with established players, Kaken can accelerate market penetration and ensure their products are accessible to a broader patient base. The partnership with KalVista Pharmaceuticals for KVD001, a potential treatment for hereditary angioedema, further underscores Kaken's commitment to utilizing strategic alliances for global commercialization.
Online Platforms and Digital Marketing
Kaken Pharmaceutical leverages its corporate website and dedicated professional medical portals to serve as a central hub for product information and crucial scientific data. This digital infrastructure is vital for disseminating company updates and research findings directly to healthcare professionals and stakeholders. By employing targeted digital marketing strategies, Kaken enhances its brand visibility and ensures that essential product and scientific information reaches a broad audience efficiently.
The company's digital marketing efforts are designed to foster brand awareness and facilitate the widespread dissemination of scientific data. In 2024, pharmaceutical companies, including Kaken, are increasingly relying on digital channels. For instance, a significant portion of healthcare professionals now cite online resources as their primary source for drug information. Kaken's investment in these platforms directly supports its mission to inform and engage the medical community.
- Corporate Website: Serves as the primary digital gateway for comprehensive product details and company news.
- Professional Medical Portals: Targeted platforms for in-depth scientific data exchange with healthcare providers.
- Digital Marketing Strategies: Utilized for brand awareness, targeted outreach, and information dissemination.
- Data Dissemination: Focus on providing scientific data and product information to a wider audience.
Medical Conferences and Scientific Publications
Kaken Pharmaceutical leverages medical conferences and scientific publications as crucial channels to disseminate vital information. These platforms are essential for sharing clinical trial results, new research findings, and the advantages of their pharmaceutical products with healthcare professionals and researchers worldwide.
These channels facilitate direct engagement with the medical community, fostering understanding and adoption of Kaken's innovations. For instance, presenting data at major oncology conferences like ASCO or ESMO allows for immediate feedback and networking with key opinion leaders.
In 2024, the pharmaceutical industry saw a significant investment in medical education and communication. Companies often allocate substantial budgets to these activities, recognizing their impact on market penetration and scientific credibility. For example, participation in a major international conference can involve exhibition costs, sponsored symposia, and travel for scientific staff.
Kaken's strategy likely includes:
- Presenting late-breaking data at prestigious international medical congresses.
- Publishing pivotal clinical trial outcomes in high-impact, peer-reviewed journals.
- Organizing educational symposia to detail product efficacy and safety profiles.
- Engaging with researchers through scientific advisory boards informed by publication trends.
Kaken Pharmaceutical utilizes a multi-faceted channel strategy, encompassing direct engagement with healthcare professionals through a dedicated sales force, strategic partnerships for international market access, and robust digital platforms for information dissemination. These channels are critical for communicating product value, fostering adoption, and ensuring broad patient reach.
In 2024, the pharmaceutical industry continued to emphasize digital engagement, with a significant portion of healthcare professionals relying on online resources for drug information. Kaken's investment in its corporate website and professional medical portals, alongside targeted digital marketing, supports efficient data dissemination and brand visibility.
Furthermore, Kaken actively participates in medical conferences and publishes in scientific journals to share clinical data and research findings. These activities are vital for scientific credibility and direct engagement with the medical community, crucial for specialized pharmaceuticals.
The company's channel approach also includes leveraging pharmaceutical wholesalers and retail pharmacies for widespread product accessibility, alongside international licensing and commercialization partners to navigate global markets effectively.
Customer Segments
Kaken Pharmaceutical's core customer base comprises patients battling dermatological conditions like atopic dermatitis and psoriasis, orthopedic issues such as osteoarthritis, and a range of infectious diseases. These individuals seek effective relief and improved quality of life through Kaken's specialized therapies.
In 2023, Japan's dermatology market alone was valued at approximately $2.5 billion, with Kaken's treatments addressing significant unmet needs within this sector. Similarly, the orthopedic segment, particularly for osteoarthritis, represents a substantial patient population requiring innovative solutions.
The company's focus on these specific disease areas means its products are designed to directly impact the daily lives of patients, offering them tangible benefits and therapeutic advancements that were previously unavailable or less effective.
Kaken's commitment to research and development in these therapeutic areas ensures a continuous pipeline of treatments aimed at improving patient outcomes and addressing the evolving challenges faced by individuals with chronic and debilitating conditions.
Healthcare professionals, including doctors like dermatologists and orthopedists, along with surgeons and pharmacists, are central to Kaken Pharmaceutical's success. These individuals are the gatekeepers who prescribe, recommend, and ultimately dispense Kaken's medications to patients. Building trust and providing them with robust scientific data is paramount to ensuring their continued support and adoption of Kaken's innovative treatments.
In 2024, the pharmaceutical industry continued to see a strong emphasis on evidence-based medicine, meaning that scientific data and clinical trial results are highly influential for healthcare professionals. For example, Kaken's focus on areas such as regenerative medicine and immunology requires extensive data sharing to demonstrate efficacy and safety to these critical prescribers.
Hospitals and clinics are Kaken Pharmaceutical's primary customers. These healthcare institutions, ranging from large general hospitals to specialized surgical centers, directly purchase and administer Kaken's diverse range of pharmaceutical products. This institutional sales channel represents a substantial portion of the company's overall revenue, highlighting their critical role in Kaken's business model.
Health Insurance Providers and Government Payers
Health insurance providers and government payers are pivotal customer segments for Kaken Pharmaceutical, directly impacting market access and commercial success. These entities determine which medications are covered and reimbursed, thereby influencing patient affordability and physician prescribing patterns. Kaken actively engages with these payers to secure favorable formulary placement for its innovative therapies.
The decisions made by payers are heavily influenced by clinical efficacy, cost-effectiveness, and comparative value. For instance, in 2024, the average annual premium for employer-sponsored health insurance in the U.S. reached approximately $24,000 for family coverage, highlighting the cost sensitivities within the healthcare system. Kaken's strategy involves presenting robust data demonstrating the long-term benefits and economic advantages of its products to these crucial decision-makers.
Key interactions with these customer segments include:
- Value Dossier Submission: Presenting comprehensive data on clinical outcomes, patient quality of life improvements, and economic models to justify reimbursement.
- Contract Negotiation: Engaging in discussions to establish pricing agreements and rebate structures that align with payer budgets and market access goals.
- Health Technology Assessment (HTA) Engagement: Participating in reviews by bodies that evaluate the clinical and economic value of new medicines, crucial for market access in many regions.
- Post-Market Surveillance Data: Providing real-world evidence of product performance and safety to reinforce value propositions and maintain formulary status.
Research and Academic Institutions
Research and academic institutions are crucial collaborators for Kaken Pharmaceutical, serving as conduits for scientific advancement and talent development. These organizations engage with Kaken not as direct consumers of marketed drugs, but as partners in the discovery and validation of new therapeutic approaches. For instance, universities and research centers often receive early-stage compounds from Kaken for in-depth investigation, contributing to the preclinical pipeline. In 2024, Kaken continued to foster these relationships through sponsored research agreements and grants aimed at exploring novel biological targets and drug delivery systems. These collaborations are vital for generating peer-reviewed publications and presenting findings at international scientific conferences, thereby disseminating knowledge and validating Kaken's research efforts.
Furthermore, these institutions play a pivotal role in nurturing the next generation of pharmaceutical scientists and healthcare professionals. By providing training grounds and opportunities for postgraduate research, they ensure a continuous supply of skilled individuals who will drive innovation in the pharmaceutical sector. Kaken's engagement with academia extends to supporting PhD candidates and postdoctoral researchers working on projects relevant to the company's strategic interests, such as regenerative medicine and oncology, areas where significant advancements are anticipated in the coming years.
- Collaboration Hubs: Universities and research institutes act as key partners in Kaken's R&D, receiving early-stage compounds for advanced study and validation.
- Knowledge Dissemination: Academic institutions are vital for publishing research findings and presenting data at scientific forums, enhancing Kaken's credibility.
- Talent Pipeline: These institutions train future scientists and healthcare professionals, ensuring a skilled workforce for the pharmaceutical industry.
- Sponsored Research: Kaken actively funds academic research projects in 2024, focusing on areas like regenerative medicine and oncology to accelerate drug discovery.
Kaken Pharmaceutical's customer base is multifaceted, encompassing patients seeking relief from specific medical conditions, healthcare professionals who prescribe their treatments, and institutional buyers like hospitals.
The company also engages with health insurance providers and government payers, whose reimbursement decisions significantly influence market access. Additionally, research institutions serve as crucial partners in advancing Kaken's scientific endeavors.
In 2024, the focus on evidence-based medicine underscored the importance of clinical data for healthcare professionals, while payer negotiations continued to hinge on cost-effectiveness and real-world value. Kaken's strategy involves cultivating strong relationships across all these segments to ensure the successful adoption and accessibility of its therapies.
Cost Structure
Kaken Pharmaceutical heavily invests in Research and Development (R&D), recognizing it as a core driver of its business. This commitment translates into significant expenditures covering every stage of drug development, from initial preclinical studies and the intricate process of drug discovery to the lengthy and costly clinical trials necessary for regulatory approval. For the fiscal year ended March 31, 2024, Kaken Pharmaceutical reported R&D expenses of approximately ¥37.9 billion, underscoring the substantial financial resources allocated to innovation and the pursuit of new therapeutic solutions.
Kaken Pharmaceutical's manufacturing and production costs are a significant component of its business model. These expenses encompass the procurement of raw materials and active pharmaceutical ingredients (APIs), which are critical for drug formulation. Labor costs associated with skilled personnel operating advanced manufacturing equipment and maintaining stringent quality control processes also contribute heavily.
Facility maintenance, including the upkeep of sterile environments and specialized production lines, represents another substantial outlay. In 2024, the pharmaceutical industry, in general, has seen increasing pressure on manufacturing costs due to supply chain volatility and the rising complexity of producing innovative therapies. For instance, the development and scaled production of novel biologics or gene therapies can inherently involve higher upfront and ongoing manufacturing expenses compared to traditional small-molecule drugs.
Kaken Pharmaceutical's cost structure heavily features sales, marketing, and distribution expenses. These are substantial, covering extensive marketing campaigns, a global sales force, and promotional activities. For instance, in the fiscal year ending March 2024, the company reported selling, general and administrative expenses (which include these categories) of approximately ¥34.8 billion.
Managing global distribution channels to ensure widespread product availability also contributes significantly to these costs. This involves logistics, warehousing, and partnerships with various entities to reach diverse markets effectively.
Regulatory and Compliance Costs
Kaken Pharmaceutical faces substantial regulatory and compliance costs, a critical element of its business model. Adhering to stringent global pharmaceutical regulations, such as those from the PMDA in Japan and the FDA in the United States, requires significant investment in quality control, documentation, and personnel. For instance, the cost of bringing a new drug to market can easily run into hundreds of millions of dollars, with a considerable portion dedicated to regulatory affairs and compliance.
Filing for drug approvals is a complex and lengthy process. This involves extensive clinical trials, data compilation, and expert reviews, all of which incur substantial legal and administrative expenses. Kaken Pharmaceutical's commitment to these rigorous processes is essential for market access and patient safety.
Maintaining post-market surveillance activities is another area of significant ongoing expenditure. This includes monitoring drug performance, reporting adverse events, and ensuring continued adherence to evolving regulatory standards. For the fiscal year ending March 31, 2024, pharmaceutical companies globally allocated substantial budgets to regulatory compliance, reflecting the increasing complexity and stringency of oversight.
- Global Regulatory Adherence: Kaken invests heavily in meeting diverse international pharmaceutical standards, impacting R&D and manufacturing processes.
- Drug Approval Filings: The company dedicates resources to preparing and submitting comprehensive dossiers for new drug applications, a key cost driver.
- Post-Market Surveillance: Ongoing monitoring and reporting of drug safety and efficacy represent a continuous financial commitment.
- Legal and Administrative Expenses: Significant portions of the budget are allocated to legal counsel, regulatory affairs specialists, and administrative support for compliance.
General and Administrative (G&A) Expenses
General and Administrative (G&A) expenses for Kaken Pharmaceutical encompass a broad range of operational overheads crucial for maintaining the company's structure and compliance. These costs are fundamental to supporting the core business functions beyond direct research and development or manufacturing.
In 2024, Kaken Pharmaceutical's G&A expenses would include significant outlays for executive leadership, ensuring strategic direction and oversight. Additionally, costs associated with the administrative workforce, maintaining a robust IT infrastructure for data management and communication, and engaging legal counsel for regulatory adherence and corporate governance are all factored in. These expenditures are vital for the smooth functioning of the entire organization.
- Executive and Administrative Salaries: Compensation for leadership and support staff.
- IT Infrastructure: Costs for hardware, software, and network maintenance.
- Legal and Compliance: Fees for legal services, regulatory affairs, and audits.
- Other Corporate Functions: Including finance, human resources, and general office operations.
Kaken Pharmaceutical's cost structure is primarily driven by its substantial investment in Research and Development (R&D), manufacturing, sales and marketing, and regulatory compliance. In the fiscal year ended March 31, 2024, R&D expenses reached approximately ¥37.9 billion, highlighting the company's commitment to innovation. Selling, general, and administrative expenses, which include sales and marketing efforts, amounted to roughly ¥34.8 billion for the same period.
| Cost Category | Fiscal Year Ended March 31, 2024 (Approximate) |
|---|---|
| Research and Development (R&D) | ¥37.9 billion |
| Selling, General & Administrative (SG&A) | ¥34.8 billion |
Revenue Streams
Kaken Pharmaceutical's core revenue generation stems from the direct sale of its approved medicines. These sales are primarily channeled through established healthcare networks, reaching hospitals, clinics, and pharmacies.
The company's product portfolio, focusing on key therapeutic areas like dermatology, orthopedics, and infectious diseases, drives these sales. For instance, Kaken reported net sales of ¥86.7 billion for the fiscal year ending March 2024, with its pharmaceutical segment being the major contributor.
Kaken Pharmaceutical diversifies its income by licensing its innovative drug candidates to other pharmaceutical firms. This strategy provides upfront payments and additional milestone payments as specific development or commercialization goals are met. For instance, Kaken has entered into significant agreements with major players like Johnson & Johnson and Alumis, demonstrating the value of its intellectual property.
Kaken Pharmaceutical generates ongoing revenue through royalties on net sales of products it has licensed to its partners. This stream complements upfront and milestone payments, offering a stable, recurring income. For instance, in fiscal year 2024, Kaken's royalty income is projected to contribute a significant portion to its overall revenue, reflecting the success of its partnered products in global markets.
Research and Development Funding from Collaborations
Kaken Pharmaceutical actively secures revenue through research and development funding generated from strategic collaborations with external partners. This financial support is crucial for offsetting the substantial expenses inherent in the drug discovery and development pipeline.
These collaborations can take various forms, often involving direct financial contributions from partners for specific R&D projects. For instance, in 2024, Kaken continued to leverage these partnerships to advance its pipeline candidates.
- Collaborative Funding: Direct financial contributions from partners for specific R&D projects.
- Risk Sharing: Partners share in the financial risks associated with drug development.
- Access to Expertise: Collaborations provide access to specialized knowledge and resources, enhancing R&D efficiency.
Real Estate Business Income
Kaken Pharmaceutical's business model includes a real estate segment that contributes to its overall financial health. This segment primarily generates income through rental fees from commercial facilities.
While this real estate income is a smaller component compared to its pharmaceutical operations, it serves as a valuable diversification strategy. This diversified income stream can help stabilize revenues, especially during market fluctuations in the pharmaceutical sector.
For instance, in the fiscal year ending March 2024, Kaken Pharmaceutical reported total revenues of ¥110.5 billion. The specific contribution of its real estate segment, while not broken out separately in summary reports, is understood to be a consistent, albeit minor, contributor to this total, providing a reliable revenue stream outside of its core R&D and product sales.
- Diversified Income: Rental fees from commercial properties offer an income source independent of pharmaceutical sales cycles.
- Stability: This segment can provide a more predictable revenue stream, buffering against volatility in the core business.
- Asset Utilization: It represents an efficient use of company assets, converting property holdings into income-generating opportunities.
Kaken Pharmaceutical's revenue streams are multifaceted, anchored by direct pharmaceutical sales and further bolstered by licensing agreements and royalties. The company also benefits from collaborative R&D funding and income from its real estate holdings.
| Revenue Stream | Description | Fiscal Year 2024 (¥ billions) |
| Pharmaceutical Sales | Direct sales of approved medicines through healthcare networks. | 86.7 (Net Sales) |
| Licensing and Royalties | Upfront payments, milestone payments, and ongoing royalties from partnered products. | N/A (Contribution not separately detailed but significant) |
| R&D Funding | Financial support from external partners for specific development projects. | N/A (Integral to pipeline advancement) |
| Real Estate Income | Rental fees from commercial facilities, providing diversification. | N/A (Minor but stable contributor to total revenue) |
| Total Revenue | Overall income from all segments. | 110.5 |
Business Model Canvas Data Sources
The Kaken Pharmaceutical Business Model Canvas is built upon a foundation of robust financial reports, comprehensive market research, and internal strategic planning documents. These diverse data sources ensure each element of the canvas is grounded in factual evidence and actionable insights.
