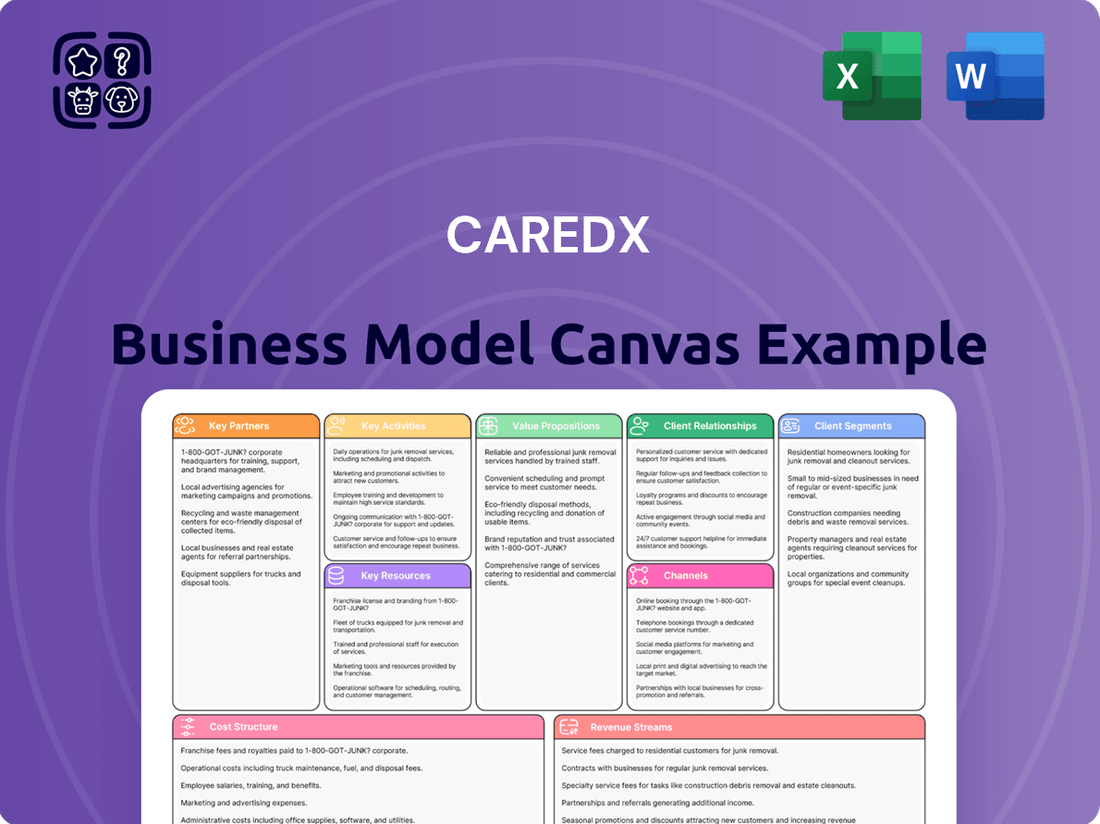
CareDx Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
CareDx Bundle
Unlock the strategic blueprint behind CareDx's success with our comprehensive Business Model Canvas. This detailed analysis breaks down how they deliver specialized transplant diagnostics, target key customer segments, and leverage strategic partnerships to drive value.
Discover CareDx's unique approach to revenue streams and cost structure, revealing the operational efficiencies and market positioning that fuel their growth. This is your chance to gain actionable insights into a leading player in the transplant diagnostics space.
Ready to dissect CareDx's winning strategy? Download the full Business Model Canvas today to gain a clear, professional overview of their entire business architecture, perfect for strategic planning and competitive analysis.
Partnerships
CareDx strategically partners with hospitals, transplant centers, and various clinics. These collaborations are key to embedding their diagnostic tools directly into patient treatment plans.
These vital relationships facilitate the widespread adoption of CareDx's tests and support their ongoing clinical validation. They also ensure patients have direct access to the company's innovative solutions.
CareDx actively partners with leading research institutions and academic centers to drive innovation in transplant diagnostics. These collaborations are crucial for conducting ongoing clinical studies, which are vital for validating new diagnostic markers and refining existing technologies. For instance, partnerships enable the development and validation of advanced assays that improve the accuracy and efficiency of post-transplant monitoring.
These academic collaborations are instrumental in building the scientific credibility and foundation for CareDx's product pipeline. By engaging with university research, the company gains access to cutting-edge scientific insights and helps shape the future of transplant care. This symbiotic relationship ensures that CareDx's offerings remain at the forefront of medical technology.
CareDx actively collaborates with biopharmaceutical companies, particularly those focused on novel immunosuppressants and treatments for transplant-related issues. These partnerships are crucial for advancing patient care and expanding the utility of CareDx's diagnostic platforms.
These strategic alliances often involve co-developing companion diagnostics that can identify patients most likely to benefit from specific therapies, thereby streamlining drug development and expediting market entry. For instance, in 2024, CareDx continued to engage with numerous pharma partners on various diagnostic development projects aimed at supporting their clinical trial pipelines.
Payers and Insurance Providers
CareDx cultivates vital relationships with both government and commercial payers to ensure reimbursement for its specialized diagnostic tests. These collaborations are fundamental for patient access to crucial transplant monitoring, which in turn fuels wider adoption and revenue predictability.
For instance, in 2023, CareDx reported that approximately 80% of its revenue was derived from commercial insurance, with Medicare and Medicaid making up the remainder. This highlights the critical importance of navigating the reimbursement landscape effectively with these key partners.
- Government Payers: Securing favorable coverage decisions and payment rates from Medicare and Medicaid is paramount for broad patient access and financial viability.
- Commercial Insurers: Establishing and maintaining contracts with a wide array of commercial insurance providers ensures that a larger patient population can afford CareDx's services.
- Reimbursement Advocacy: Proactive engagement with payers to demonstrate the clinical utility and cost-effectiveness of transplant diagnostics is key to securing and maintaining reimbursement.
Technology and Digital Health Platforms
CareDx's key partnerships with technology and digital health platforms are crucial for seamless integration into healthcare systems. Collaborations with electronic medical record (EMR) providers, such as EPIC, are vital. These partnerships allow CareDx's digital solutions and test results to be directly embedded into existing clinical workflows, improving data accessibility and patient management for transplant teams.
These integrations streamline the process for healthcare providers, ensuring that critical transplant information is readily available. For instance, the ability to directly access CareDx's transplant monitoring data within an EMR system enhances the efficiency of care delivery and supports more informed decision-making by physicians.
- EMR Integration: Partnerships with EMR vendors facilitate the direct flow of transplant patient data, including test results, into hospital systems.
- Digital Health Platforms: Collaborations extend to broader digital health ecosystems, enabling comprehensive patient monitoring and management.
- Workflow Enhancement: These integrations are designed to improve the operational efficiency of transplant centers by reducing manual data entry and improving data accessibility.
CareDx's strategic alliances with biopharmaceutical companies are critical for advancing transplant care. These collaborations often focus on developing companion diagnostics that identify patients likely to respond to specific therapies, thereby accelerating drug development and market entry. In 2024, CareDx continued its engagement with numerous pharmaceutical partners on diagnostic projects supporting their clinical trial pipelines, enhancing the precision of treatment selection.
| Partnership Type | Focus Area | Impact | Example/Data Point (2024) |
|---|---|---|---|
| Biopharmaceutical Companies | Companion Diagnostics Development | Accelerated drug development, improved patient selection for therapies | Ongoing collaborations on diagnostic projects supporting clinical trial pipelines |
| Hospitals & Transplant Centers | Diagnostic Integration | Widespread adoption of tests, direct patient treatment plans | Key channel for embedding diagnostic tools into clinical workflows |
| Research Institutions | Innovation & Validation | Development of new assays, scientific credibility | Crucial for clinical studies validating new diagnostic markers |
| Payers (Government & Commercial) | Reimbursement & Access | Ensuring patient access to monitoring, revenue predictability | ~80% of 2023 revenue from commercial insurance, highlighting payer importance |
| EMR/Digital Health Platforms | Data Integration & Workflow | Seamless data flow, improved clinical efficiency | Direct integration into EMRs like EPIC for enhanced data accessibility |
What is included in the product
CareDx's business model focuses on providing innovative solutions for transplant patients, leveraging its proprietary testing platforms and digital health services to improve patient outcomes and streamline care pathways.
This model is designed to capture value through a combination of direct sales of diagnostic tests, recurring revenue from digital health subscriptions, and strategic partnerships within the healthcare ecosystem.
CareDx's Business Model Canvas offers a clear, one-page snapshot of their strategy, simplifying complex operations for stakeholders.
It effectively addresses the pain point of understanding intricate business strategies by condensing CareDx's approach into a digestible format for quick review and comparison.
Activities
CareDx's primary focus is on the ongoing research and development of innovative molecular diagnostic assays and digital tools specifically designed for organ transplant recipients. This commitment is evident in their advancement of a robust pipeline, including the significant AlloHeme trial, which is exploring new diagnostic capabilities for hematologic malignancies.
Commercialization and Sales for CareDx involves actively marketing, selling, and distributing their specialized diagnostic products and testing services. Their primary customers are transplant centers and healthcare providers, crucial for reaching patients needing advanced organ transplant monitoring.
A significant emphasis is placed on expanding their sales footprint. This strategic push aims to increase the volume of testing services utilized, directly impacting revenue growth and market penetration for their innovative solutions.
In 2024, CareDx reported strong performance in their commercialization efforts, with their flagship AlloSure® and AlloMap® testing services seeing continued adoption. This expansion is directly contributing to their revenue streams, which are vital for reinvestment in research and development.
CareDx operates high-complexity clinical laboratories, processing patient samples for advanced molecular diagnostic testing. This is crucial for delivering accurate and timely results, particularly for their flagship products like AlloSure and AlloMap, which are vital for transplant patient management.
Maintaining stringent quality control and adhering to regulatory compliance, such as CLIA and CAP, are paramount to the integrity and reliability of these laboratory operations. In 2023, CareDx reported revenue from its testing services business, which includes these laboratory operations, reaching $228.5 million, highlighting the significant scale of their clinical lab activities.
Data Analysis and Software Development
CareDx focuses on developing and maintaining sophisticated software tools and digital platforms. These are crucial for managing transplant patients, enabling remote monitoring, and performing in-depth data analytics. The company is committed to improving integration with Electronic Medical Record (EMR) systems, aiming to create smoother clinical workflows for healthcare providers.
This commitment to software development is evident in their ongoing investments. For instance, in 2023, CareDx reported that its digital health solutions, which include these software platforms, were a significant contributor to its revenue, underscoring the importance of this key activity. The company continues to refine its offerings, with a strategic emphasis on enhancing data interoperability and user experience within the transplant care ecosystem.
- Software Development: Creating and updating proprietary software for patient management and data analysis.
- Platform Maintenance: Ensuring the reliability and security of digital platforms supporting transplant care.
- EMR Integration: Enhancing connectivity with existing Electronic Medical Record systems to improve data flow.
- Data Analytics Enhancement: Continuously improving the capabilities of their analytical tools for better patient insights.
Regulatory Affairs and Reimbursement Management
CareDx actively navigates the intricate regulatory pathways to secure approvals for its innovative diagnostic tests, a critical step for market access. This involves meticulous preparation and submission of data to bodies like the FDA, ensuring compliance with evolving standards.
Ensuring adequate payer coverage and favorable reimbursement rates is paramount for CareDx's commercial success. The company engages with insurance providers and government payers to demonstrate the clinical utility and economic value of its tests, aiming to drive average selling price (ASP) growth. In 2023, CareDx reported that approximately 90% of its revenue was generated from covered lives, highlighting the importance of reimbursement management.
- Regulatory Approvals: Securing FDA clearance and CE marking for key diagnostic solutions like AlloSure and AlloMap.
- Payer Engagement: Negotiating with Medicare, private payers, and integrated delivery networks to establish favorable coverage policies.
- Reimbursement Strategy: Implementing strategies to optimize reimbursement rates and expand access to testing services.
- ASP Growth: Focusing on demonstrating value to drive higher average selling prices for their transplant diagnostic portfolio.
CareDx's key activities revolve around continuous innovation in molecular diagnostics for organ transplant patients, focusing on research and development of new assays and digital tools. This includes advancing their pipeline, such as the AlloHeme trial for hematologic malignancies. They also concentrate on the commercialization and sales of their specialized testing services, primarily targeting transplant centers and healthcare providers, with a strategic goal to expand their market footprint and increase testing volumes.
Full Version Awaits
Business Model Canvas
The CareDx Business Model Canvas preview you're viewing is the actual document you will receive upon purchase. This is not a sample or a mockup, but a direct representation of the comprehensive analysis you'll gain access to. Once your order is complete, you'll download this exact file, providing you with a ready-to-use strategic framework for CareDx.
Resources
CareDx's proprietary molecular diagnostic technologies, specifically their patented donor-derived cell-free DNA (dd-cfDNA) and gene expression profiling platforms, are central to their business model. These advanced technologies underpin their flagship AlloSure and AlloMap tests, offering a significant competitive advantage in the transplant diagnostics market.
These unique technological assets enable CareDx to provide highly accurate and non-invasive monitoring solutions for transplant recipients. For instance, AlloSure, which utilizes dd-cfDNA technology, achieved a substantial revenue contribution for CareDx, with the company reporting approximately $145 million in AlloSure revenue for the full year 2023, highlighting the commercial success driven by this proprietary technology.
CareDx's extensive clinical data and published studies are foundational. These resources, including real-world evidence, are crucial for demonstrating the efficacy of their diagnostic solutions to healthcare providers and insurance companies. For instance, data supporting their AlloSure Kidney Transplant product has been instrumental in securing payer coverage, with studies showing improved patient management and reduced adverse events.
CareDx's business model hinges on its network of state-of-the-art CLIA-certified and CAP-accredited laboratories. These facilities are critical for accurately processing patient samples, underpinning the reliability of their diagnostic testing services. As of late 2023, CareDx operates multiple such advanced laboratories strategically located to serve their patient base efficiently.
Skilled Scientific and Medical Personnel
CareDx relies heavily on its skilled scientific and medical personnel, including molecular biologists, geneticists, and bioinformaticians, to drive its research and development efforts. This specialized team is fundamental to creating and refining the company's innovative diagnostic tests. Their collective expertise ensures the accuracy and efficacy of the services offered, directly impacting patient outcomes and the company's competitive edge.
The company's clinical operations and test development are directly supported by a cadre of medical professionals and experienced laboratory technicians. These individuals are essential for maintaining high standards in sample processing, data analysis, and quality control. In 2024, CareDx continued to invest in attracting and retaining top talent in these fields, recognizing that their proficiency is a cornerstone of the business model.
- Expertise in Molecular Biology and Genetics: Crucial for developing and validating advanced diagnostic assays.
- Bioinformatics Capabilities: Essential for analyzing complex genomic data and identifying actionable insights.
- Clinical Operations Proficiency: Ensures reliable and efficient processing of patient samples and delivery of test results.
- Commitment to Innovation: A highly skilled workforce fuels the continuous improvement and expansion of CareDx's product pipeline.
Intellectual Property (Patents and Trademarks)
CareDx's intellectual property, particularly its patents and trademarks, forms a critical cornerstone of its business model. This robust protection shields its innovative diagnostic tests and proprietary technologies, creating a substantial barrier for potential competitors and solidifying its market standing. As of early 2024, CareDx held a significant number of issued patents and pending applications, underscoring its commitment to innovation and market exclusivity.
The company's intellectual property strategy is designed to safeguard its competitive advantage in the transplant diagnostics space. This includes protecting its AlloSure and AlloMap testing platforms, which are central to its revenue generation. The strength of its patent portfolio directly impacts its ability to maintain premium pricing and market share.
- Patents: CareDx maintains a substantial portfolio of patents covering its core transplant diagnostic technologies, including cell-free DNA analysis and related methods.
- Trademarks: Key brand names like AlloSure and AlloMap are trademarked, ensuring brand recognition and preventing market confusion.
- Trade Secrets: Proprietary algorithms and manufacturing processes are protected as trade secrets, further fortifying its competitive moat.
- Market Protection: This IP framework is crucial for preventing infringement and maintaining CareDx's leadership in post-transplant patient monitoring.
CareDx's key resources are its proprietary molecular diagnostic technologies, extensive clinical data, a network of CLIA-certified laboratories, and a highly skilled workforce. These assets, protected by a robust intellectual property portfolio, enable the company to offer specialized transplant diagnostics.
The company's technological foundation, particularly its dd-cfDNA and gene expression profiling platforms, underpins its leading diagnostic tests like AlloSure and AlloMap. This technological edge, coupled with significant investment in R&D, drives its market position.
CareDx's clinical data and published studies are vital for demonstrating test efficacy and securing payer coverage. For example, data supporting AlloSure Kidney Transplant has been instrumental in this regard.
The company's CLIA-certified and CAP-accredited laboratories are essential for accurate sample processing, ensuring the reliability of its diagnostic services. As of late 2023, CareDx operated multiple such advanced facilities to efficiently serve its patient base.
A skilled team of molecular biologists, geneticists, and bioinformaticians is crucial for R&D and test refinement, directly impacting patient outcomes and competitive advantage. In 2024, CareDx continued to focus on attracting and retaining this specialized talent.
CareDx's intellectual property, including patents and trademarks for its core technologies, creates a significant barrier to entry for competitors. This IP strategy is key to maintaining market exclusivity and premium pricing for its transplant diagnostic solutions.
| Key Resource | Description | Impact on Business Model | 2023/2024 Data Point |
|---|---|---|---|
| Proprietary Technologies | dd-cfDNA and gene expression profiling | Underpins AlloSure and AlloMap tests, creating competitive advantage. | AlloSure revenue ~ $145 million in 2023. |
| Clinical Data & Studies | Real-world evidence and published research | Demonstrates efficacy, aids payer coverage. | Data supports AlloSure Kidney Transplant product. |
| Laboratory Network | CLIA-certified and CAP-accredited facilities | Ensures accurate sample processing and reliable test delivery. | Operated multiple advanced labs as of late 2023. |
| Skilled Personnel | Molecular biologists, geneticists, bioinformaticians | Drives R&D, test refinement, and innovation. | Continued investment in talent acquisition in 2024. |
| Intellectual Property | Patents and trademarks | Protects innovations, creates market exclusivity and barriers to entry. | Held significant number of issued patents and pending applications by early 2024. |
Value Propositions
CareDx's diagnostic tests, including AlloSure and AlloMap, offer a critical advantage by detecting organ rejection early and non-invasively. This allows for prompt treatment, which can significantly improve patient health and the longevity of transplanted organs.
For instance, studies have shown that using AlloSure in kidney transplant patients can lead to earlier identification of rejection episodes compared to traditional methods. This early warning system empowers clinicians to act faster, potentially preventing irreversible damage to the graft and enhancing patient survival rates.
The ability to detect rejection before significant damage occurs is a key value proposition. In 2024, the focus on improving transplant outcomes continues, and CareDx's technology plays a vital role in achieving this by providing actionable data for personalized patient management.
CareDx's diagnostic solutions provide crucial information about organ health, leading to better patient management and improved quality of life for transplant recipients.
By minimizing the need for invasive procedures like biopsies, CareDx directly contributes to enhancing the overall well-being and health outcomes of these patients.
In 2023, CareDx's transplant diagnostics business generated approximately $337 million in revenue, underscoring the demand for their solutions that improve patient care.
CareDx's diagnostic solutions are designed to significantly lower healthcare expenditures within transplantation programs. By preventing unnecessary hospitalizations and mitigating irreversible organ damage, these tools directly address costly complications.
For instance, the company's post-transplant surveillance solutions can help avert costly readmissions. In 2024, the average cost of a hospital readmission for transplant patients can range from $15,000 to $30,000, a figure CareDx's early detection capabilities aim to reduce.
Furthermore, by enabling more precise treatment guidance, their diagnostics help optimize medication and intervention strategies, thereby avoiding expensive, ineffective therapies and improving long-term patient outcomes, which translates to substantial savings for healthcare systems.
Comprehensive Transplant Care Pathway Support
CareDx offers a complete support system for the entire transplant journey, from before the procedure to long after. This includes vital testing services, innovative digital health tools, and dedicated patient solutions, creating a truly integrated and holistic approach to transplant care.
This comprehensive offering aims to improve patient outcomes and streamline the complex transplant process. For instance, their AlloSure Kidney test, a key component, provides critical information for managing kidney transplant recipients. In 2023, CareDx reported significant revenue from its testing services, demonstrating the market's reliance on these integrated solutions.
The value proposition is built on providing seamless support across multiple touchpoints:
- Pre-transplant assessment and risk stratification.
- Post-transplant monitoring and management of organ health.
- Digital tools for patient engagement and data tracking.
- Solutions designed to improve long-term graft survival and patient quality of life.
Evidence-Based and Clinically Differentiated Solutions
CareDx provides healthcare solutions that are not only clinically differentiated but also strongly supported by scientific evidence. This commitment to data-driven development ensures that clinicians can trust the efficacy and value of these offerings, fostering greater confidence in their use.
The company's focus on clinical validation is key to its success in the transplant community. By demonstrating tangible benefits through rigorous research, CareDx drives the adoption of its innovative products and services.
- Clinically Validated Offerings: CareDx's solutions are built upon a foundation of robust clinical evidence, ensuring high performance and reliability.
- Differentiated Value: The company's products offer unique advantages that set them apart in the healthcare market, particularly within transplant care.
- Evidence-Based Approach: A strong emphasis on scientific research and data underpins all of CareDx's developments, building trust with healthcare professionals.
- Driving Adoption: This commitment to evidence and differentiation directly translates into increased acceptance and utilization by clinicians and healthcare systems.
CareDx's value proposition centers on delivering superior patient outcomes and operational efficiencies in organ transplantation. Their diagnostic tests, like AlloSure and AlloMap, enable early, non-invasive detection of organ rejection, allowing for timely interventions that preserve graft function and enhance patient survival. This proactive approach not only improves quality of life but also mitigates significant healthcare costs associated with managing complications and readmissions.
The company provides a comprehensive suite of solutions spanning the entire transplant continuum, from pre-transplant risk assessment to post-transplant monitoring. This integrated offering, supported by digital tools for patient engagement, streamlines care pathways and fosters better long-term management. In 2023, CareDx's transplant diagnostics business generated approximately $337 million in revenue, reflecting strong market demand for their evidence-based, clinically differentiated solutions.
CareDx's commitment to clinical validation ensures that their products offer tangible benefits, building trust and driving adoption among healthcare professionals. For example, early detection of rejection episodes using AlloSure in kidney transplant patients can prevent irreversible damage, a critical factor in improving graft longevity. The financial impact is substantial, as averting costly readmissions, which can range from $15,000 to $30,000 in 2024, directly contributes to cost savings for healthcare systems.
| Value Proposition | Key Benefit | Supporting Data/Example |
|---|---|---|
| Early & Non-Invasive Rejection Detection | Improved graft survival, better patient outcomes | AlloSure enables earlier identification of rejection episodes. |
| Comprehensive Transplant Support | Streamlined care, enhanced patient engagement | Integrated testing, digital tools, and patient solutions. |
| Reduced Healthcare Costs | Mitigation of costly complications and readmissions | Averting readmissions can save $15,000-$30,000 per transplant patient (2024 estimate). |
| Clinically Validated Offerings | High performance, reliability, and trust | Evidence-based approach drives adoption and confidence. |
Customer Relationships
CareDx cultivates deep connections with transplant centers and their clinicians by providing specialized clinical support teams. These teams offer ongoing training and educational programs, ensuring healthcare professionals can effectively utilize and interpret CareDx's diagnostic tests. This commitment to education and support is crucial for building lasting, collaborative partnerships within the transplant community.
CareDx provides personalized patient support programs designed to empower transplant recipients in managing their post-transplant health. These initiatives offer crucial assistance, fostering better adherence to treatment regimens and promoting overall well-being.
Leveraging digital tools and remote monitoring services, CareDx aims to enhance patient engagement and provide continuous support. This approach allows for proactive health management, ultimately improving outcomes for individuals who have undergone transplantation.
CareDx cultivates deep relationships with key opinion leaders and researchers through active participation in collaborative research and development projects. These partnerships are crucial for driving innovation in transplant diagnostics.
Joint publications and presentations at leading scientific conferences solidify CareDx's position as an industry leader. For instance, in 2023, CareDx presented numerous abstracts and posters at major transplant and immunology conferences, showcasing advancements in their testing platforms.
This engagement not only fosters a spirit of scientific inquiry but also ensures that CareDx's diagnostic solutions are at the forefront of clinical practice. Their commitment to R&D is reflected in the ongoing development of new assays and the expansion of their product pipeline, directly benefiting the transplant community.
Account Management for Healthcare Systems
CareDx assigns dedicated account managers to each healthcare system, fostering deep understanding of their unique operational challenges and strategic goals. These managers act as liaisons, ensuring seamless integration of CareDx's diagnostic solutions and driving adoption for optimal patient care and hospital efficiency. In 2024, CareDx continued to emphasize EMR integration as a key component of its account management strategy.
- Dedicated Account Management: Specialists are assigned to transplant centers and hospital systems.
- Needs Assessment & Integration: Account managers work to understand specific client needs and integrate CareDx solutions effectively.
- Value Optimization: Focus on maximizing the benefits clients receive from CareDx's product suite.
- Strategic Operations: Including EMR integration initiatives to streamline workflows and data management.
Transparent Communication with Payers
CareDx prioritizes open and transparent communication with insurance providers and government payers. This approach is crucial for showcasing the clinical utility and cost-effectiveness of their diagnostic tests. By clearly demonstrating value, CareDx aims to secure favorable coverage decisions and robust reimbursement policies.
- Demonstrating Value: CareDx actively communicates the clinical benefits and economic advantages of its transplant diagnostics to payers.
- Reimbursement Success: In 2024, CareDx continued to engage with payers to ensure broad access to its testing solutions for transplant patients.
- Policy Advocacy: The company works to influence coverage policies, aiming to align reimbursement with the demonstrated clinical need for advanced diagnostics.
CareDx builds strong relationships with transplant centers through dedicated account management and clinical support teams, offering training and integration services. They also focus on patient empowerment with personalized support programs and digital tools for better health management. Strategic partnerships with researchers and active engagement in scientific conferences further solidify their industry leadership and drive innovation.
| Relationship Type | Key Activities | Engagement Focus | 2024 Highlight |
|---|---|---|---|
| Transplant Centers/Clinicians | Dedicated Account Management, Clinical Support, Training Programs | Effective utilization of diagnostics, workflow integration | Emphasis on EMR integration for seamless data management |
| Transplant Recipients | Personalized Support Programs, Digital Tools, Remote Monitoring | Patient adherence, health management, improved outcomes | Continued development of patient-centric digital platforms |
| Researchers/KOLs | Collaborative R&D, Joint Publications, Conference Presentations | Driving innovation, scientific validation | Showcasing advancements in transplant diagnostics at major conferences |
| Payers/Insurance Providers | Value Communication, Policy Advocacy | Securing favorable coverage, ensuring patient access | Continued engagement with payers to demonstrate clinical utility and cost-effectiveness |
Channels
CareDx leverages a dedicated direct sales force to cultivate relationships with key stakeholders in transplant medicine, including physicians, surgeons, and hospital administrators. This direct engagement is crucial for educating these professionals about their specialized diagnostic solutions and fostering product adoption within transplant centers.
In 2024, CareDx reported that their direct sales force was instrumental in driving the adoption of their AlloSure and AlloMap testing, which are vital for post-transplant patient management. This hands-on approach allows for in-depth product demonstrations and tailored discussions on how their offerings can improve patient outcomes and operational efficiency for transplant programs.
CareDx leverages its own CLIA-certified laboratories, a cornerstone of its direct-to-customer model for testing services. This direct approach allows for rigorous quality control and aims for efficient turnaround times, crucial for patient care and physician confidence.
In 2024, CareDx continued to refine its laboratory operations, focusing on scalability and advanced diagnostic capabilities. The company's commitment to in-house processing directly impacts its ability to manage data integrity and the speed at which critical results are delivered to healthcare providers and patients.
CareDx's digital health platforms and software integrations are key to its business model, enabling seamless data flow and enhanced accessibility for healthcare professionals. By integrating with Electronic Medical Record (EMR) systems, CareDx streamlines workflows, allowing for quicker access to critical patient data and test results.
These integrations are crucial for efficient patient management and clinical decision-making. For instance, in 2024, the company continued to expand its partnerships with major EMR providers, aiming to make its transplant patient management solutions more readily available within existing healthcare IT infrastructures.
Medical Conferences and Scientific Publications
CareDx leverages medical conferences and scientific publications as key channels to reach and educate the transplant community. These platforms are essential for sharing their latest research and data. In 2024, CareDx continued its active participation in prominent transplant conferences, showcasing advancements in their organ care testing solutions.
Presenting research findings at these events, such as the American Society of Transplantation (AST) Annual Meeting, allows CareDx to directly engage with nephrologists, transplant surgeons, and other key opinion leaders. Publications in high-impact, peer-reviewed journals further validate their technology and build credibility within the scientific and medical fields, driving adoption of their products.
- Conference Participation: CareDx actively presents at major transplant society meetings globally, fostering direct engagement with clinicians.
- Data Dissemination: Research data on patient outcomes and test performance is shared through oral and poster presentations.
- Peer-Reviewed Publications: Findings from clinical studies are published in leading journals to establish scientific validation and awareness.
Patient Advocacy Groups and Online Communities
CareDx actively engages with patient advocacy groups and online communities, fostering direct connections with transplant recipients. This approach is crucial for building brand awareness and establishing trust within this vital patient population. For instance, in 2024, CareDx continued its partnerships with numerous patient organizations, amplifying its reach and support services.
These collaborations serve as a powerful channel for CareDx to disseminate information about its diagnostic solutions and patient support programs. By participating in community discussions and events, the company gains invaluable insights into patient needs and challenges. This direct feedback loop is instrumental in informing and refining CareDx's product development pipeline. In 2024, feedback from these communities directly influenced the development of enhanced patient-facing educational materials.
The benefits extend to:
- Direct Patient Engagement: Reaching transplant patients effectively to offer support and information.
- Brand Trust and Awareness: Building credibility and recognition within the patient community.
- Product Development Insights: Gathering real-world patient feedback to guide innovation.
- Community Support: Providing resources and a sense of belonging for transplant recipients.
CareDx utilizes a multi-faceted channel strategy, combining direct sales, digital platforms, and community engagement to reach its target audience. This approach ensures comprehensive market penetration and effective communication of its specialized transplant diagnostics.
In 2024, CareDx's direct sales force remained a primary channel, fostering deep relationships with transplant centers and driving the adoption of key products like AlloSure and AlloMap. The company's CLIA-certified labs operate as a direct channel for its testing services, emphasizing quality control and rapid turnaround times. Furthermore, digital health platforms and EMR integrations streamline data access for healthcare professionals, a critical component of their 2024 strategy to enhance workflow efficiency.
| Channel | Description | 2024 Focus/Impact |
|---|---|---|
| Direct Sales Force | Personalized engagement with physicians, surgeons, and hospital administrators. | Drove adoption of AlloSure and AlloMap; educated on product benefits for patient outcomes. |
| CLIA-Certified Laboratories | In-house processing of diagnostic tests. | Ensured quality control and efficient turnaround times for critical patient results. |
| Digital Health Platforms & EMR Integrations | Streamlining data flow and accessibility for healthcare professionals. | Expanded EMR partnerships to integrate transplant patient management solutions into existing IT infrastructures. |
| Medical Conferences & Publications | Disseminating research and engaging with the transplant community. | Active participation in conferences like AST Annual Meeting to showcase advancements. |
| Patient Advocacy Groups & Online Communities | Direct engagement with transplant recipients. | Built brand awareness and trust; gathered patient feedback to inform product development. |
Customer Segments
Transplant nephrologists and cardiologists are pivotal to CareDx's business. These specialists oversee the complex care of patients who have received kidney, heart, and lung transplants. Their expertise directly influences which diagnostic tools are utilized for patient monitoring.
As the primary prescribers, their adoption of CareDx's surveillance and rejection monitoring tests is critical. In 2024, the demand for advanced transplant diagnostics continues to grow, driven by the increasing number of transplant procedures and the need for more precise patient management.
Transplant surgeons are central to CareDx’s business model, influencing both initial adoption and ongoing product use. They are directly involved in the transplantation procedure itself and then manage patients long-term, making diagnostic insights crucial for post-operative care and monitoring organ health. For example, CareDx’s AlloSure Kidney Monitor, a key product, provides cell-free DNA testing to help surgeons assess transplant health.
Transplant centers and hospitals are key partners for CareDx, integrating their diagnostic tests and digital tools directly into patient care. For instance, in 2023, CareDx's AlloSure and AlloMap tests were utilized by a significant number of transplant centers across the United States, supporting critical decisions in organ health monitoring.
These healthcare institutions leverage CareDx's offerings to enhance patient outcomes and streamline transplant management. The company's solutions are designed to fit within existing clinical workflows, providing actionable data for physicians.
Patients and Caregivers
Transplant patients and their caregivers, while not the direct payers, are the core beneficiaries of CareDx's innovations. Their lived experience and desire for improved health outcomes and quality of life significantly shape the demand for advanced monitoring solutions. This segment actively seeks ways to better manage their post-transplant journey, influencing the adoption of new technologies.
Their influence is crucial as they are the ultimate users of the diagnostic tools and services. For instance, patients and caregivers are often the ones advocating for more frequent or comprehensive testing to ensure the long-term success of a transplant. This personal stake drives their engagement with healthcare providers regarding monitoring strategies.
- Patient Empowerment: Patients and caregivers seek tools that provide clear, actionable information about transplant health, fostering a sense of control and involvement in their care.
- Quality of Life Focus: This segment prioritizes solutions that minimize invasiveness and maximize the potential for a normal, active life post-transplant.
- Information Seeking: They actively research and inquire about the latest advancements in transplant monitoring, often bringing new technologies to the attention of their medical teams.
Biopharmaceutical Companies
Biopharmaceutical companies represent a key customer segment for CareDx, actively seeking collaborations. These companies are increasingly interested in leveraging CareDx's specialized knowledge in transplant immunology to advance their drug development pipelines. For instance, in 2024, the biopharma sector continued to invest heavily in precision medicine, a field where CareDx's diagnostic capabilities are highly relevant.
Partnerships with CareDx offer biopharmaceutical firms significant advantages in clinical trial support. This includes access to CareDx's established patient registries and expertise in identifying and recruiting appropriate patient cohorts for transplant-related studies. The demand for such specialized support is high, as evidenced by the growing complexity and cost of clinical trials in recent years.
Furthermore, biopharmaceutical companies are keen to utilize CareDx's companion diagnostics. These diagnostics are crucial for identifying patients who are most likely to benefit from specific therapies, thereby improving trial success rates and accelerating drug approvals. CareDx's growing portfolio of transplant-specific tests positions them as a valuable partner in this endeavor.
The strategic value for biopharma partners lies in accessing CareDx's deep understanding of transplant rejection mechanisms and immune response. This can lead to the development of novel therapeutics and improved treatment strategies for transplant recipients. By 2024, the focus on post-transplant monitoring and management had intensified, creating a fertile ground for such collaborations.
- Growing Interest in Transplant Immunology: Biopharma firms are increasingly partnering with CareDx to tap into its specialized expertise in transplant immunology for drug discovery and development.
- Clinical Trial Support: These companies value CareDx's ability to provide robust support for clinical trials, including patient recruitment and data management in the transplant space.
- Companion Diagnostics Integration: Biopharmaceutical clients seek to integrate CareDx's diagnostic solutions to identify patient populations likely to respond to their targeted therapies.
- Accelerated Drug Development: Collaborations aim to leverage CareDx's insights to accelerate the development and approval of novel treatments for transplant patients.
CareDx's customer segments are primarily healthcare professionals and institutions involved in organ transplantation, along with biopharmaceutical companies. Transplant physicians, including nephrologists and cardiologists, are crucial as they prescribe diagnostic tests. Surgeons also play a vital role in adopting and utilizing these tools for patient care.
Transplant centers and hospitals integrate CareDx's solutions into their workflows to improve patient outcomes. Patients and their caregivers are the ultimate beneficiaries, driving demand for better monitoring. Biopharmaceutical companies partner with CareDx for clinical trial support and companion diagnostics, leveraging its transplant immunology expertise.
| Customer Segment | Role in Business Model | Key Needs/Interactions |
| Transplant Physicians (Nephrologists, Cardiologists) | Prescribers and key influencers of diagnostic tool adoption. | Accurate, actionable data for patient monitoring and rejection assessment. |
| Transplant Surgeons | Directly involved in procedures and post-operative care management. | Tools to monitor organ health and assess transplant success. |
| Transplant Centers & Hospitals | Integrators of diagnostic tests into clinical practice. | Seamless workflow integration, improved patient outcomes, operational efficiency. |
| Transplant Patients & Caregivers | End beneficiaries, advocating for effective monitoring. | Improved quality of life, clear information, proactive health management. |
| Biopharmaceutical Companies | Partners for drug development and clinical trials. | Clinical trial support, patient stratification, companion diagnostics, transplant immunology insights. |
Cost Structure
Research and Development (R&D) represents a substantial cost for CareDx, reflecting their commitment to innovation in transplant diagnostics. A significant portion of these expenses is channeled into developing new diagnostic assays and conducting crucial clinical validation studies. For instance, in 2023, R&D expenses amounted to $111.2 million, a notable increase from $93.4 million in 2022, underscoring the ongoing investment in their pipeline of solutions for various organ transplants.
Clinical laboratory operations represent a significant cost for CareDx, encompassing the expenses tied to their high-complexity testing facilities. These costs include substantial outlays for skilled personnel like scientists and technicians, essential reagents and consumables for testing, and the maintenance of sophisticated laboratory equipment.
Furthermore, robust quality control measures, crucial for ensuring test accuracy and regulatory compliance, add to the operational expenditure. Facility overhead, covering rent, utilities, and compliance with stringent laboratory standards, also forms a key component of this cost structure.
CareDx's cost structure heavily features sales and marketing expenses, crucial for reaching its target audience of transplant centers and pathologists. These costs include maintaining a direct sales force, which is a significant investment in personnel and training.
Marketing campaigns, both digital and traditional, are essential for building brand awareness and educating the market about CareDx's diagnostic solutions. The company also allocates substantial resources to attending major medical conferences, providing a platform to showcase innovations and engage directly with key opinion leaders.
Building and nurturing relationships with healthcare providers and payers is another core component of their sales and marketing strategy, requiring ongoing engagement and support. For instance, in the first quarter of 2024, CareDx reported sales and marketing expenses of $51.2 million, highlighting the significant investment in these areas.
General and Administrative Expenses
General and administrative expenses encompass the essential corporate functions that keep CareDx running smoothly. This includes costs like executive salaries, accounting, human resources, IT systems, and legal support. These are the backbone costs that enable the company's specialized operations.
CareDx has demonstrated a strategic focus on optimizing these overheads. For instance, the company has actively worked to reduce its legal expenditures, a significant component of G&A. This focus on efficiency aims to bolster profitability by controlling costs that aren't directly linked to product development or service delivery.
- Corporate Overhead: Includes executive compensation, finance, HR, and IT support.
- Legal and Compliance: Covers regulatory affairs, litigation, and corporate governance.
- Administrative Staff: Salaries and benefits for non-R&D, non-operational personnel.
- IT Infrastructure: Costs associated with maintaining and upgrading technology systems.
Regulatory and Reimbursement Compliance Costs
CareDx incurs significant expenses to navigate the intricate landscape of healthcare regulations and reimbursement. These costs are essential for ensuring their diagnostic tests meet stringent quality standards and are recognized by payers. For instance, maintaining compliance with the Clinical Laboratory Improvement Amendments (CLIA) and obtaining FDA approvals for new assays represent substantial outlays.
The company must also invest in teams and systems to manage the complex process of securing and maintaining reimbursement from various insurance providers. This includes coding, billing, and appealing denied claims, which are critical for revenue generation. In 2023, the healthcare industry, in general, saw increased scrutiny on compliance, potentially leading to higher spending in this area for companies like CareDx.
- Regulatory Compliance: Expenses associated with CLIA, FDA submissions, and other governmental regulations.
- Reimbursement Management: Costs for billing, coding, claims processing, and payer negotiations.
- Quality Assurance: Investments in maintaining laboratory accreditations and test validation processes.
- Legal and Consulting Fees: Payments for expert advice on navigating complex compliance and reimbursement pathways.
CareDx's cost structure is dominated by research and development, clinical laboratory operations, and sales and marketing efforts. These areas represent significant investments necessary for innovation, service delivery, and market penetration. For example, in 2023, R&D expenses were $111.2 million, while Q1 2024 sales and marketing costs reached $51.2 million, illustrating the substantial financial commitment to these crucial functions.
| Cost Category | 2023 Expense (Millions USD) | Q1 2024 Expense (Millions USD) | Key Components |
|---|---|---|---|
| Research & Development | 111.2 | N/A | New assay development, clinical validation |
| Clinical Laboratory Operations | N/A | N/A | Personnel, reagents, equipment, quality control |
| Sales & Marketing | N/A | 51.2 | Sales force, marketing campaigns, conference attendance |
| General & Administrative | N/A | N/A | Executive salaries, finance, HR, IT, legal |
| Regulatory & Reimbursement | N/A | N/A | Compliance (CLIA, FDA), reimbursement management |
Revenue Streams
CareDx's core revenue originates from its molecular diagnostic testing services, primarily AlloSure and AlloMap. These tests are crucial for monitoring transplant patients, covering kidney, heart, and lung recipients.
In 2023, CareDx reported testing revenue of $238.9 million. This significant figure underscores the company's reliance on its diagnostic solutions for patient surveillance in the transplant community.
CareDx generates significant revenue through the sale of its diagnostic products, primarily reagents and kits, to clinical laboratories and research institutions. This core segment consistently demonstrates growth, reflecting the increasing demand for advanced transplant diagnostics.
In 2023, CareDx reported product revenue of $224.4 million, a notable increase from $184.6 million in 2022. This upward trend highlights the market's adoption of their specialized testing solutions.
Patient and Digital Solutions Revenue is a key component of CareDx's business model, encompassing income from their digital health platforms and specialized software tools designed for transplant management. These offerings streamline patient care and administrative processes within transplant centers.
This segment also generates revenue through remote patient monitoring services, allowing for continuous oversight of transplant recipients, and through transplant pharmacy services, which cater to the unique medication needs of this patient population. This diversification strengthens their revenue base.
The company reported significant year-over-year growth in this area. For instance, in the first quarter of 2024, CareDx's Patient and Digital Solutions segment saw its revenue increase by 16% compared to the same period in 2023, reaching $36.4 million.
Pharma Services Revenue
CareDx generates revenue from its pharma services by partnering with biopharmaceutical companies. These collaborations involve providing specialized diagnostic services and expertise crucial for advancing clinical trials and drug development programs.
This revenue stream is built on CareDx's deep understanding of transplant immunology and its advanced testing capabilities. For instance, in 2023, the company reported significant growth in its pharma services segment, driven by an increasing number of partnerships aimed at identifying patient populations and monitoring treatment efficacy.
- Diagnostic Services for Clinical Trials: CareDx offers its proprietary testing platforms to biopharma clients for use in their clinical studies, helping to stratify patients and assess treatment responses.
- Drug Development Support: The company provides scientific expertise and data analysis to aid in the development and regulatory approval of new therapies for transplant patients.
- Partnership Growth: Revenue from this segment is directly tied to the number and scale of ongoing collaborations with pharmaceutical and biotechnology firms.
Strategic Partnerships and Licensing
CareDx can generate revenue through strategic partnerships and licensing its proprietary technologies. This involves agreements where other companies pay upfront fees, milestone payments tied to development progress, or ongoing royalties based on the commercial success of licensed intellectual property.
These collaborations allow CareDx to leverage its innovations across a broader market. For instance, in 2023, CareDx continued to explore and engage in partnerships aimed at expanding the reach and application of its transplant diagnostics. While specific financial figures for licensing revenue are often embedded within broader partnership disclosures, the company's focus on innovation suggests a significant revenue potential from these avenues.
- Licensing Fees: Upfront payments for the right to use CareDx's patented technologies.
- Milestone Payments: Payments triggered upon the achievement of specific development or commercial targets by a partner.
- Royalties: A percentage of sales revenue generated by products or services that incorporate CareDx's licensed technology.
CareDx's revenue streams are primarily built on its molecular diagnostic testing services, with AlloSure and AlloMap being the flagship offerings for transplant patient monitoring. The company also generates substantial income from the sale of diagnostic products, including reagents and kits, to laboratories and research institutions.
Diversification is evident in their Patient and Digital Solutions segment, which includes revenue from digital health platforms and remote patient monitoring, showing strong growth with a 16% increase in Q1 2024. Furthermore, Pharma Services revenue is driven by collaborations with biopharmaceutical companies for clinical trials and drug development, a segment that saw significant growth in 2023 due to increased partnerships.
Strategic partnerships and technology licensing also contribute to CareDx's revenue through upfront fees, milestone payments, and royalties, underscoring a multifaceted approach to monetizing its innovations.
| Revenue Stream | Description | 2023 Revenue (Millions USD) | 2022 Revenue (Millions USD) | Year-over-Year Growth |
|---|---|---|---|---|
| Testing Services | AlloSure, AlloMap for transplant monitoring | 238.9 | N/A | N/A |
| Product Sales | Reagents and kits for labs | 224.4 | 184.6 | 21.6% |
| Patient & Digital Solutions | Digital health platforms, remote monitoring | N/A | N/A | 16% (Q1 2024 vs Q1 2023) |
| Pharma Services | Clinical trials, drug development support | N/A | N/A | Significant growth in 2023 |
| Partnerships & Licensing | Technology licensing, collaborations | N/A | N/A | Potential for significant revenue |
Business Model Canvas Data Sources
The CareDx Business Model Canvas is built upon a foundation of robust financial disclosures, comprehensive market research reports, and strategic insights gleaned from industry analysis. These data sources ensure each component of the canvas accurately reflects the company's current operations and future potential.
