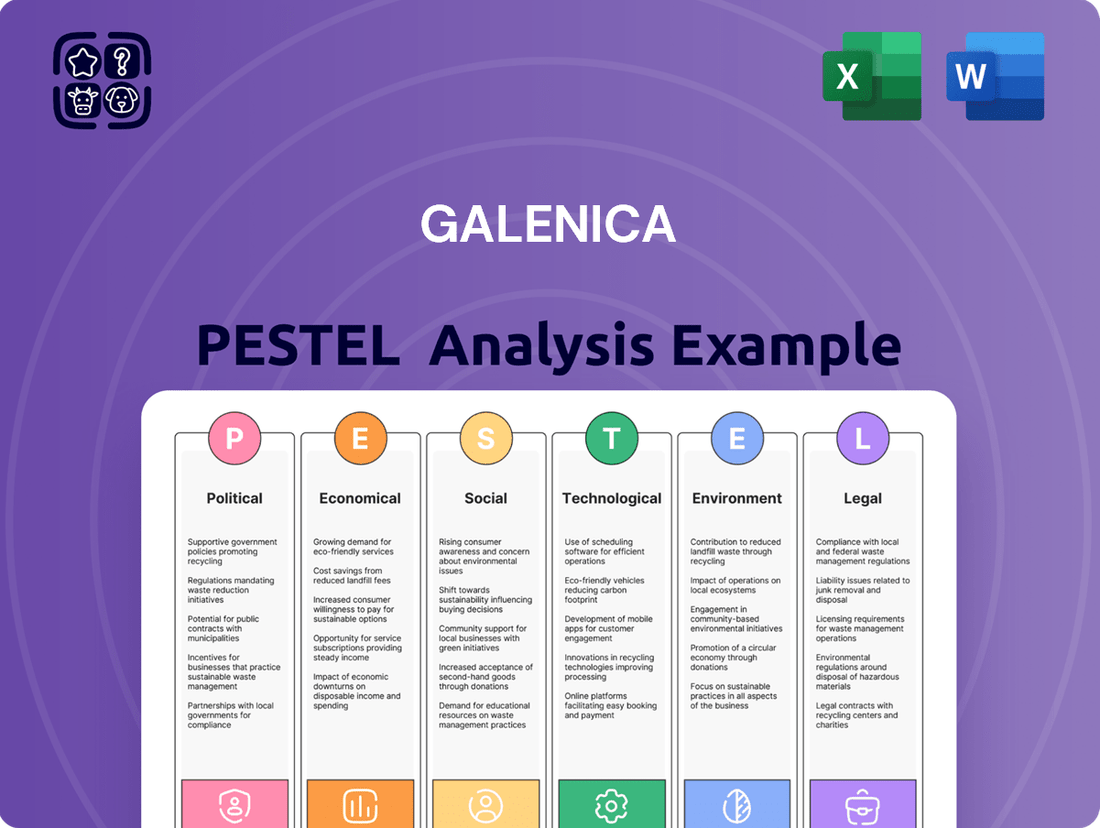
Galenica PESTLE Analysis
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Galenica Bundle
Unlock the strategic landscape surrounding Galenica with our meticulously crafted PESTLE analysis. Understand the critical political, economic, social, technological, legal, and environmental factors that are shaping its present and future. This comprehensive report offers actionable intelligence, perfect for investors, strategists, and anyone seeking a competitive edge.
Don't get left behind by evolving market dynamics. Our PESTLE analysis provides you with the foresight to anticipate challenges and capitalize on opportunities within the Galenica ecosystem. Gain a deep understanding of the external forces at play.
Equip yourself with the knowledge to make informed decisions. Whether you're assessing investment potential or planning your next strategic move, this PESTLE analysis is your essential guide.
Ready to elevate your understanding of Galenica's operating environment? Purchase the full PESTLE analysis now and gain immediate access to expert-driven insights.
Political factors
Swiss governmental healthcare policies, such as those managed by the Federal Office of Public Health (FOPH), actively shape Galenica's operating environment. The government's focus on cost containment and universal access directly impacts drug pricing and reimbursement frameworks. For instance, reforms intended to streamline drug approvals and pricing, like those seen in the 2024 amendments to the Health Insurance Ordinance (KVV), aim to control overall healthcare spending.
The Healthcare Benefit Ordinance (KLV/OPre) updates, particularly those effective January 1, 2025, signal a continued governmental drive towards efficiency and transparency in healthcare services. These revisions often involve adjustments to how new and existing medications are reimbursed, affecting Galenica's ability to secure favorable pricing and market access for its pharmaceutical products. The government's ongoing efforts to manage expenditure are a critical political factor for Galenica's wholesale and retail pharmacy businesses.
Switzerland's drug pricing and reimbursement framework, overseen by the Federal Office of Public Health (FOPH), directly impacts Galenica's business. Changes like the shift from public price to ex-factory price (FAP) for listed drugs, alongside foreign price referencing, can alter Galenica's wholesale and pharmacy margins. For instance, in 2024, ongoing FOPH reviews for the Specialty List (SL) continue to scrutinize the cost-effectiveness of new pharmaceutical products, influencing market access and potential sales volumes for Galenica's portfolio.
Switzerland boasts a consistently stable political environment, a significant advantage for Galenica, as it fosters predictability and minimizes geopolitical uncertainties in its operational planning. This stability is a bedrock for long-term investment and strategic development.
However, the nation's federalist system, where powers are distributed among federal, cantonal, and municipal levels, introduces complexity. Healthcare, in particular, sees shared responsibilities, meaning national policies may be implemented differently across cantons. For instance, in 2024, while the federal government sets overarching healthcare regulations, cantons retain considerable autonomy in licensing healthcare facilities and approving pricing for certain services, necessitating localized compliance strategies.
Intellectual Property Protection
Switzerland's commitment to strong intellectual property (IP) protection is a significant advantage for Galenica, particularly in its health and beauty sector. This robust legal framework, which includes stringent patent and trademark laws, is vital for companies investing heavily in research and development. The Swiss legal system provides a secure environment, encouraging innovation and ensuring market exclusivity for new products.
Galenica benefits directly from these protections, which safeguard its investments in developing unique formulations and branding for its health and beauty lines. For instance, the Swiss Federal Institute of Intellectual Property reported a 5.2% increase in patent applications in 2023 compared to the previous year, underscoring the active innovation landscape. This legal certainty allows Galenica to confidently pursue groundbreaking product development.
- Strong Patent Enforcement: Switzerland's rigorous enforcement of patents protects Galenica's exclusive rights to innovative pharmaceutical and health products, fostering continued R&D investment.
- Trademark Security: The country's well-defined trademark laws safeguard Galenica's brands, preventing counterfeiting and dilution of its market presence in the competitive health and beauty industry.
- Legal Stability: The predictable and stable Swiss legal system provides a secure foundation for Galenica's long-term product development strategies and market positioning.
- R&D Incentive: Robust IP protection acts as a powerful incentive for Galenica to allocate resources towards the creation of novel health and beauty solutions, knowing its intellectual assets are well-defended.
International Trade Agreements and Harmonization
While Galenica operates primarily within Switzerland, its international trade agreements and regulatory harmonization efforts can still have an impact. Switzerland's ongoing trade relations, particularly with the European Union, are crucial. The revision of the Swiss Therapeutic Products Act (TPA) is a significant development, aiming to bring Swiss regulations closer to those of the EU, such as the EU-Medical Device Regulation (MDR) and the EU-In Vitro Diagnostic Regulation (IVDR). This alignment could streamline market access and reduce complexities for Galenica’s product development and wholesale operations.
The harmonization process, expected to continue through 2024 and into 2025, is designed to foster greater legal clarity and reduce existing trade barriers. For Galenica, this could mean smoother cross-border logistics and a more predictable regulatory environment for specific therapeutic products. The goal is to facilitate the flow of goods and services, potentially enhancing Galenica's ability to source materials or distribute products across European markets.
- Regulatory Alignment: Switzerland's TPA revision aims to match EU standards, including MDR and IVDR, by 2025.
- Trade Barrier Reduction: Harmonization efforts are intended to decrease complexities in international trade for medicinal products.
- Market Access: Closer alignment with EU regulations could improve Galenica's access to broader European markets for certain product lines.
- Legal Clarity: The updated TPA is expected to provide a more defined and predictable legal framework for therapeutic products.
Swiss governmental healthcare policies, like those from the Federal Office of Public Health (FOPH), significantly shape Galenica's operational landscape, with a consistent focus on cost containment and universal access influencing drug pricing and reimbursement. Revisions to ordinances, such as the Healthcare Benefit Ordinance (KLV/OPre) effective January 1, 2025, underscore this drive for efficiency, directly impacting Galenica's wholesale and retail pharmacy margins through adjustments in how medications are reimbursed and market access is secured.
Switzerland's stable political climate provides a predictable environment for Galenica's long-term investments and strategic planning, minimizing geopolitical risks. However, the federalist structure introduces complexity, as cantons retain autonomy in healthcare implementation, requiring localized compliance strategies for Galenica to navigate varying regulations.
Robust intellectual property (IP) protection in Switzerland, demonstrated by a 5.2% increase in patent applications in 2023, is crucial for Galenica's R&D investments, safeguarding its innovations in pharmaceuticals and health products through strong patent and trademark enforcement.
Switzerland's ongoing efforts to align its Therapeutic Products Act (TPA) with EU regulations, including MDR and IVDR by 2025, aim to reduce trade barriers and streamline market access for Galenica's products within broader European markets.
What is included in the product
Galenica's PESTLE analysis dissects the external macro-environmental factors influencing the company across Political, Economic, Social, Technological, Environmental, and Legal dimensions.
This comprehensive evaluation provides actionable insights for strategic decision-making and identifying market opportunities.
Galenica's PESTLE Analysis offers a streamlined, easily digestible summary, eliminating the pain of wading through lengthy reports for quick strategic discussions.
Economic factors
Healthcare spending in Switzerland is on an upward trend, with projections indicating it will surpass CHF 100 billion by 2026. This means per-person spending could approach CHF 11,600.
Several factors contribute to this increase, including a growing elderly population and the introduction of innovative, albeit often costly, medical treatments. This persistent rise in expenditure directly impacts health insurance premiums.
For 2025, insurance premiums are anticipated to climb by an average of 6%. While this signifies a robust and expanding market for healthcare providers and associated industries, it simultaneously creates affordability challenges for both individuals and insurance companies.
Switzerland's robust economy directly impacts consumer purchasing power for Galenica's offerings. In 2024, Swiss household disposable income remained strong, bolstered by low unemployment rates, which generally hover around 2%. This financial stability allows consumers to allocate more funds towards health and beauty products and over-the-counter medications.
When the Swiss economy experiences growth, Galenica likely sees increased demand for its premium and non-essential health and wellness items. For instance, during periods of economic expansion, consumers are more inclined to spend on higher-margin beauty products or specialized health supplements, benefiting Galenica's retail segments.
Conversely, any economic downturn or slowdown in Switzerland could shift consumer priorities. During such times, there's a tendency for consumers to focus spending on essential pharmaceutical needs, potentially reducing discretionary purchases of beauty or wellness products. This highlights the direct correlation between the nation's economic health and Galenica's sales performance in various product categories.
Inflationary pressures directly impact Galenica by increasing the cost of essential goods, from finished pharmaceutical products to the raw materials needed for its own branded medications. These rising costs extend to operational expenses throughout its extensive pharmacy network and sophisticated logistics infrastructure.
Effectively managing these escalating supply chain and distribution costs is paramount for Galenica to safeguard its profit margins. For instance, while Galenica reported robust sales growth of 4.7% in its 2024 financial year, maintaining cost efficiency remains a critical focus area amidst these inflationary headwinds.
Swiss Franc Exchange Rate Fluctuations
The exchange rate of the Swiss Franc (CHF) plays a crucial role in Galenica's financial performance. A stronger franc can lower the cost of imported pharmaceuticals and raw materials, which is beneficial for its wholesale business. For instance, if the CHF strengthens against the Euro, Galenica's costs for sourcing medicines from the Eurozone would decrease.
Conversely, a robust CHF can make Galenica's own-brand products, such as those from its Verfora segment, more expensive for international buyers. This could impact the competitiveness and sales volume of its exports. In 2024, Galenica reported positive organic growth for its export business, including brands like Verfora, indicating that other factors, possibly including favourable regulatory shifts, helped offset potential currency headwinds.
- Import Cost Reduction: A stronger CHF can directly reduce the cost of imported goods for Galenica.
- Export Competitiveness Impact: A stronger CHF can make Galenica's exported products more expensive internationally.
- 2024 Export Performance: Galenica's export segment, including Verfora, demonstrated positive organic growth in 2024.
- Currency Hedging: Galenica likely employs strategies to mitigate the negative impacts of CHF fluctuations on its international sales.
Market Growth in Pharmaceutical and Healthcare Sector
The Swiss pharmaceutical market is demonstrating robust expansion, with forecasts suggesting a significant increase. Projections estimate the market will grow from an estimated USD 3.3 billion in 2024 to USD 5.7 billion by 2033.
This upward trend is largely fueled by breakthroughs in biopharmaceuticals and a sustained commitment to research and development within Switzerland. Such advancements are creating a fertile ground for innovation and product launches.
The overall growth trajectory of the pharmaceutical sector presents a favorable economic climate for Galenica. This environment offers substantial opportunities for the company to achieve continued sales increases and expand its market presence across both its wholesale and retail operations.
- Projected Market Value: Expected to reach USD 5.7 billion by 2033, up from USD 3.3 billion in 2024.
- Key Growth Drivers: Advancements in biopharmaceuticals and a strong emphasis on R&D.
- Impact on Galenica: Favorable conditions for sales growth and market share expansion in wholesale and retail.
Switzerland's economic stability underpins consumer demand for Galenica's diverse product portfolio. With low unemployment, projected at around 2% for 2024, and strong disposable incomes, consumers are well-positioned to purchase health and beauty items. This economic resilience translates to sustained revenue potential for Galenica, particularly in its retail segments offering higher-margin goods.
Inflationary pressures are a key consideration, as rising costs for raw materials and operational expenses can impact Galenica's profitability. For example, while the company achieved 4.7% sales growth in its 2024 financial year, managing these escalating costs is crucial for margin protection. The exchange rate of the Swiss Franc also plays a dual role, potentially lowering import costs while increasing the price of exported goods.
The Swiss pharmaceutical market is experiencing significant growth, projected to increase from USD 3.3 billion in 2024 to USD 5.7 billion by 2033, driven by biopharmaceutical advancements and R&D investment. This expansion creates a favorable environment for Galenica's wholesale and retail operations, offering opportunities for increased sales and market penetration.
| Economic Factor | 2024/2025 Data/Projection | Impact on Galenica |
|---|---|---|
| Swiss Economic Growth | Strong consumer purchasing power, low unemployment (~2%) | Sustained demand for health and beauty products |
| Inflation | Rising costs for raw materials and operations | Pressure on profit margins, need for cost efficiency |
| Swiss Franc Exchange Rate | Fluctuations impacting import/export costs | Potential for reduced import costs, but increased export prices |
| Pharmaceutical Market Growth | Projected USD 3.3B (2024) to USD 5.7B (2033) | Opportunities for sales growth and market expansion |
Same Document Delivered
Galenica PESTLE Analysis
The preview shown here is the exact document you’ll receive after purchase—fully formatted and ready to use. This Galenica PESTLE analysis delves into the Political, Economic, Social, Technological, Legal, and Environmental factors impacting the pharmaceutical industry. It provides a comprehensive overview crucial for strategic planning and market understanding.
Sociological factors
Switzerland's demographic shift towards an older population is a significant trend. By 2024, projections indicate a continued rise in the proportion of citizens over 65, reaching approximately 21% of the total population. This aging demographic naturally correlates with a higher incidence of chronic diseases, such as cardiovascular conditions, diabetes, and respiratory ailments. Consequently, there's a robust and growing demand for pharmaceutical products, long-term care services, and specialized healthcare solutions to manage these ongoing health needs.
Galenica is strategically positioned to capitalize on these societal shifts. Its extensive network of pharmacies across Switzerland, coupled with its strong wholesale distribution capabilities, allows it to effectively serve this expanding elderly market. The company can readily meet the increasing demand for prescription medications required for chronic disease management. Furthermore, Galenica's healthcare services, including vital offerings like vaccinations and health screenings, are becoming increasingly essential for maintaining the well-being of an aging populace, contributing to sustained revenue growth.
Societal trends are increasingly highlighting health awareness and preventive care within the Swiss population. This growing emphasis on well-being and proactive health management presents a significant opportunity for Galenica. The company's pharmacies are well-positioned to capitalize on this shift by expanding their offerings in health services and expert advice.
Consumers are also showing a greater inclination towards self-medication and readily available health solutions. Galenica can leverage this by broadening its selection of over-the-counter (OTC) health and beauty products, catering to the demand for accessible personal healthcare. This aligns with the evolving consumer behavior towards taking more personal responsibility for their health.
The impact of these trends is already evident in Galenica's performance. In 2024, a notable 39% increase in customers sought healthcare services and advice at Galenica pharmacies was observed. Furthermore, demand for vaccinations saw a substantial rise of 20%, underscoring the effectiveness of adapting to these growing health consciousness trends.
Consumers are increasingly prioritizing natural, sustainable, and personalized options in the health and beauty sector. This shift is a significant sociological factor impacting companies like Galenica. For instance, a 2023 Nielsen report indicated that 73% of global consumers would change their purchasing habits to reduce their environmental impact, highlighting the demand for eco-friendly products.
Galenica's success hinges on its capacity to innovate and market its own health and beauty lines that resonate with these evolving consumer demands. Failing to adapt could lead to a loss of market share. The market for natural and organic personal care products alone was valued at over $55 billion globally in 2023 and is projected to grow steadily.
This trend also directly shapes the product selections available within Galenica's extensive pharmacy network. Pharmacies are becoming destinations for curated wellness, and stocking brands that reflect ethical sourcing and clean ingredients is becoming a competitive necessity. In 2024, beauty retailers reported a significant uptick in sales for products featuring transparent ingredient lists and eco-conscious packaging.
Digital Health Adoption by Consumers
Consumers are increasingly comfortable with digital health solutions, expecting seamless online interactions for everything from accessing health information to managing prescriptions. This shift is driven by convenience and a growing reliance on technology in daily life. For instance, a 2024 survey indicated that over 60% of patients would prefer to receive e-prescriptions directly to their smartphone.
Galenica's strategy directly addresses this by developing a comprehensive digital medication journey. This initiative aims to connect the entire process, from a doctor’s digital prescription to the patient receiving and managing their medication, thereby enhancing user experience and adherence. The company is investing in platforms that facilitate e-prescriptions and offer patients clear overviews of their ongoing treatments.
The societal trend favors greater patient empowerment through technology. By 2025, it's projected that more than 70% of pharmacies will offer digital prescription management services. This aligns with Galenica's focus on providing transparent prescription overviews, a key feature for consumers seeking more control over their healthcare.
Key aspects of digital health adoption impacting Galenica include:
- Growing consumer demand for telehealth and remote monitoring services.
- Increased comfort with online pharmacies and digital prescription services.
- Expectation of personalized health information accessible via digital platforms.
- A rising preference for integrated digital health solutions that streamline the patient journey.
Public Trust in Healthcare Providers and Pharmacies
Public trust in Swiss healthcare providers, including pharmacists, is a cornerstone for Galenica's operations. This high level of confidence, consistently demonstrated in surveys, translates directly into customer loyalty within its extensive pharmacy network. For instance, a 2023 study by the Swiss Federal Statistical Office indicated that over 80% of the Swiss population expressed high trust in their local pharmacies for health advice. This trust is a critical competitive differentiator, especially in the retail segment where Galenica's pharmacies are often the first point of contact for many health concerns.
Maintaining this trust hinges on Galenica's commitment to quality service, readily available expert advice from pharmacists, and unwavering adherence to ethical practices. These elements are crucial for retaining customers and reinforcing the pharmacies' role as accessible, reliable healthcare hubs. The importance of this accessibility was highlighted in early 2024 when Galenica reported a 5% year-on-year increase in foot traffic across its pharmacies, partly attributed to their role in providing accessible health screenings and advice.
- High Trust Levels: Swiss citizens consistently rank pharmacists among the most trusted healthcare professionals, a key asset for Galenica.
- Foundation for Loyalty: Maintaining trust through excellent service and expert guidance is vital for customer retention and competitive edge.
- Accessible Healthcare Hubs: Pharmacies serve as crucial, easily accessible points for health advice and services within communities.
- Demonstrated Engagement: Increased pharmacy visits in early 2024 suggest a growing reliance on these accessible health touchpoints.
Societal factors significantly influence Galenica's market position. The Swiss population is aging, with those over 65 projected to be around 21% by 2024, increasing demand for chronic disease management products and services. Furthermore, a growing emphasis on health awareness and preventive care drives demand for Galenica's pharmacy services and OTC products. For example, in 2024, Galenica saw a 39% increase in customers seeking healthcare services at its pharmacies.
Technological factors
The digitalization of pharmacy operations is a major technological driver, with advancements like electronic shelf labels and integrated management systems significantly boosting efficiency and customer experience. Galenica is leading this charge with its 'digital medication journey,' aiming for a smooth transition from doctor's prescription to patient delivery, incorporating easy e-prescribing and clear prescription tracking.
This technological shift extends to optimizing logistics and digital ordering within Galenica's wholesale operations, streamlining the supply chain. For instance, by mid-2024, over 80% of pharmacies in key European markets were expected to have some form of electronic prescription system in place, a trend Galenica is leveraging.
The increasing adoption of e-prescriptions and electronic patient records (EPR) across Switzerland marks a crucial technological advancement in healthcare. Galenica's extensive pharmacy network, encompassing brands like Amavita, Coop Vitality, and Sun Store, is fully equipped to process these digital prescriptions.
This digital transformation enhances patient safety by reducing prescription errors and improves the efficiency of medication management. Furthermore, it fosters better interoperability, allowing seamless data sharing among various healthcare professionals and institutions. For example, by mid-2024, the Swiss government reported a significant increase in the daily volume of e-prescriptions processed nationwide, indicating strong user and provider uptake.
Automation and artificial intelligence are transforming logistics, promising significant efficiency gains and cost reductions for Galenica’s distribution operations. By integrating these technologies, Galenica can streamline its supply chain, leading to faster delivery times and more accurate inventory management.
The joint venture Health Supply with Planzer has shown promising growth throughout 2024, particularly in optimizing pharmaceutical logistics and implementing sustainable transport. This collaboration is a key example of how Galenica is leveraging technological advancements to enhance its distribution network.
Furthermore, Galexis is actively improving operational efficiency by bundling its services, a strategy that likely incorporates automated processes and data-driven insights. This focus on service optimization directly benefits from the advancements in AI and automation within the logistics sector.
Telemedicine and Digital Health Platforms
The growing adoption of telemedicine and digital health platforms is fundamentally reshaping healthcare delivery, directly impacting Galenica. These advancements offer a significant opportunity to broaden patient reach and enhance service accessibility, but they also necessitate a strategic shift in how pharmacies interact with consumers. For instance, in 2024, the global telemedicine market was valued at over $120 billion, with projections indicating continued robust growth, highlighting the scale of this digital transformation.
Galenica must actively integrate its offerings with these burgeoning digital health ecosystems. This could involve partnerships with telehealth providers or developing proprietary digital solutions that streamline prescription management and medication adherence for patients utilizing remote consultations. Failing to adapt risks leaving Galenica behind as patients increasingly expect seamless digital health experiences.
Key considerations for Galenica include:
- Integration Capabilities: Developing or acquiring technology to connect with major telemedicine platforms for prescription fulfillment and patient data sharing.
- Digital Service Expansion: Exploring the launch of a Galenica-branded digital health app offering services like virtual pharmacist consultations, medication reminders, and online refills.
- Data Security and Privacy: Ensuring robust compliance with regulations like GDPR and HIPAA when handling sensitive patient health information within digital platforms.
- Patient Engagement Models: Adapting traditional pharmacy services to complement digital interactions, fostering loyalty in a hybrid care environment.
Data Security and Cybersecurity
Galenica's increasing reliance on digital platforms necessitates a strong focus on data security and cybersecurity. Protecting sensitive patient information is crucial for maintaining trust and complying with evolving regulations. The company invests in advanced technologies to shield its IT infrastructure from the growing landscape of cyber threats.
Compliance with stringent data protection laws, such as GDPR or equivalent regional mandates, is a significant technological factor. Galenica actively addresses this by implementing robust security protocols and regularly updating its systems. Employee training is also a key component, with the company conducting multiple data protection and IT security training sessions annually to ensure its workforce remains vigilant.
- Data Breach Costs: The average cost of a data breach in the healthcare sector reached $10.10 million in 2023, highlighting the financial implications of inadequate security.
- Cybersecurity Investment: Global spending on cybersecurity solutions is projected to reach $234.7 billion in 2024, reflecting the industry-wide emphasis on digital defense.
- Employee Training Effectiveness: A significant percentage of cyber incidents are attributed to human error, underscoring the importance of Galenica's multi-annual training initiatives.
Technological advancements are profoundly reshaping Galenica's operational landscape, from digitized pharmacy workflows to sophisticated logistics. The widespread adoption of e-prescriptions, with over 80% of pharmacies in key European markets expected to utilize them by mid-2024, underscores this shift. Galenica's network, including Amavita and Sun Store, is poised to leverage this digital infrastructure for enhanced efficiency and patient safety.
Legal factors
Galenica's operations are deeply shaped by Switzerland's robust pharmaceutical legal landscape, anchored by the Therapeutic Products Act (TPA). This legislation, along with numerous ordinances, dictates crucial aspects of the pharmaceutical industry, from initial marketing authorization and rigorous quality control to responsible advertising and distribution channels.
Compliance with these stringent rules is paramount, ensuring product safety and efficacy but also presenting significant operational hurdles. For instance, the TPA's detailed requirements for Good Manufacturing Practice (GMP) and pharmacovigilance necessitate substantial investment in quality assurance systems.
Recent legislative updates, particularly those aligning the TPA with European Union directives, reflect an ongoing effort to harmonize standards and bolster patient safety. These revisions, effective from 2022, introduced stricter requirements for clinical trials and the oversight of medical devices, impacting market access and R&D strategies.
The Swiss Agency for Therapeutic Products (Swissmedic) plays a central role in enforcing these regulations, reviewing product applications and conducting market surveillance. In 2023, Swissmedic reported approving over 1,500 new medicinal product applications, highlighting the dynamic nature of regulatory approvals.
Galenica operates under stringent data privacy laws like the Swiss Federal Act on Data Protection (FADP), which mirrors GDPR guidelines. This means meticulous handling of sensitive patient information across its pharmacies and digital health platforms is paramount. Failure to comply can result in significant fines and damage to reputation. For instance, GDPR violations can lead to fines of up to €20 million or 4% of global annual turnover.
Galenica operates within strict competition laws, particularly in Switzerland, to ensure fair market practices. As a major player in pharmacy and wholesale distribution, its actions, including strategic investments such as increasing its stake in Redcare Pharmacy, are closely monitored to prevent monopolistic behavior.
The Swiss Competition Commission (COMCO) actively scrutinizes market dominance. For instance, in 2023, COMCO investigated several sectors for potential anti-competitive practices, emphasizing the need for companies like Galenica to demonstrate that their market strategies foster, rather than hinder, competition.
Galenica's growth strategies, including mergers and acquisitions, must be carefully structured to comply with merger control regulations. Failure to do so could result in significant fines or divestment orders, impacting its market position and profitability.
Employment and Labor Laws
Swiss employment and labor laws significantly shape Galenica's operations, influencing everything from employee contracts to daily work conditions. Adherence to these regulations is paramount for Galenica's workforce, which spans its retail pharmacies, extensive distribution networks, and central corporate offices. This includes strict compliance with rules governing standard working hours, minimum wage requirements, mandated employee benefits, and robust workplace safety protocols.
Galenica's commitment to fostering a positive work environment is underscored by its focus on diversity and the reduction of workplace hazards. For instance, in 2024, Swiss companies generally saw a slight uptick in reported occupational accidents, making Galenica's proactive safety initiatives even more critical. The company actively works to minimize the incidence of occupational accidents and illnesses, ensuring a safe and healthy workplace for all its employees, a key factor in operational stability and employee retention.
Key legal considerations for Galenica include:
- Working Hours and Overtime: Adherence to the Swiss Labour Act (ArG) concerning maximum weekly hours and overtime regulations is crucial.
- Wages and Benefits: Ensuring fair wages and compliance with statutory employee benefits, such as social security contributions and vacation entitlements, is a legal necessity.
- Workplace Safety: Implementing and maintaining safety standards as mandated by Swiss law to prevent accidents and occupational diseases is a continuous effort.
- Non-discrimination and Equal Opportunity: Upholding principles of equal treatment and non-discrimination in hiring, promotion, and compensation practices is a fundamental legal requirement.
Product Liability and Consumer Protection
Galenica operates under stringent product liability laws, meaning the company is accountable for ensuring the safety and effectiveness of the pharmaceuticals it distributes, as well as its own health and beauty product lines. This responsibility extends to rigorous quality control and adherence to manufacturing standards.
Consumer protection legislation further governs Galenica's operations by mandating fair advertising, accurate product labeling, and transparent sales practices. For instance, the EU's General Data Protection Regulation (GDPR) impacts how Galenica handles customer data, emphasizing privacy and consent, a key aspect of consumer trust.
In 2024, the pharmaceutical industry saw increased scrutiny on drug safety, with regulatory bodies like the FDA issuing recalls and fines for non-compliance. Galenica's commitment to these regulations is vital for avoiding costly legal battles and protecting its brand reputation. For example, in 2023, fines for product liability violations in the healthcare sector globally exceeded billions of dollars, underscoring the financial risks involved.
Adherence to these legal frameworks is not just about avoiding penalties; it's fundamental to maintaining consumer confidence, particularly in the health and wellness sector. Galenica's proactive approach to compliance, including robust internal audits and staff training on consumer protection laws, helps mitigate legal risks and fosters long-term customer loyalty.
- Product Safety: Galenica is liable for any harm caused by defective pharmaceutical products it distributes or its own branded goods.
- Advertising Standards: Consumer protection laws require truthful and non-misleading claims in all marketing and advertising materials.
- Labeling Requirements: Accurate and comprehensive labeling, including ingredients, usage instructions, and warnings, is legally mandated.
- Regulatory Compliance: Staying abreast of evolving regulations, such as those related to data privacy and product recalls, is critical for mitigating legal exposure.
Galenica's legal framework is significantly influenced by Swiss and international regulations governing pharmaceuticals, data privacy, competition, and employment. Compliance with these laws is essential for maintaining operational integrity and market standing.
The company must navigate stringent product liability and consumer protection laws, ensuring the safety and accuracy of its offerings. This includes meticulous adherence to advertising standards and product labeling requirements to foster consumer trust and avoid legal repercussions.
Swiss employment laws dictate workplace conditions, wages, and safety protocols, requiring Galenica to maintain high standards for its workforce across all operational sites. Proactive safety measures are crucial, especially given industry trends in occupational accidents.
Galenica's strategic moves, including acquisitions and market practices, are subject to scrutiny by the Swiss Competition Commission to prevent anti-competitive behavior, underscoring the need for careful planning and regulatory adherence.
| Legal Area | Key Regulations/Acts | Impact on Galenica | Example/Data Point (2023-2024) |
|---|---|---|---|
| Pharmaceuticals | Therapeutic Products Act (TPA), EU Directives | Marketing authorization, quality control, R&D | Swissmedic approved over 1,500 new medicinal product applications in 2023. |
| Data Privacy | Swiss Federal Act on Data Protection (FADP), GDPR | Handling of sensitive patient information | GDPR fines up to €20 million or 4% of global annual turnover for non-compliance. |
| Competition | Swiss Competition Act | Market practices, mergers, acquisitions | Swiss Competition Commission (COMCO) actively scrutinizes market dominance. |
| Employment | Swiss Labour Act (ArG) | Working hours, wages, safety, non-discrimination | Focus on minimizing occupational accidents in 2024 due to slight industry uptick. |
| Product Liability & Consumer Protection | Product Liability Laws, Consumer Protection Laws | Product safety, advertising, labeling | Global fines for product liability violations in healthcare exceeded billions of dollars in 2023. |
Environmental factors
Growing environmental consciousness and increasingly stringent rules for corporate sustainability are shaping Galenica's business, especially concerning its supply chain and how it makes its products. These factors are becoming more important for the company's long-term success.
Both the European Union and Switzerland are set to implement tougher environmental sustainability rules in 2025. These new regulations will likely focus on making drug production more eco-friendly, improving packaging materials, and enhancing waste management practices across the industry.
Galenica already has a framework in place with its Environmental Code of Conduct, demonstrating a commitment to responsible environmental practices. A key objective is the reduction of greenhouse gas emissions stemming from its day-to-day operations.
Galenica's diverse operations, from pharmaceutical manufacturing to pharmacy retail, inevitably produce a range of waste streams. These include specialized pharmaceutical waste requiring careful handling, extensive packaging materials from product distribution, and general operational waste from its widespread network of pharmacies and logistics hubs.
Navigating the complex landscape of environmental regulations is crucial for Galenica. Efficient waste management and robust recycling initiatives are not just about compliance; they are key drivers of the company's overall environmental performance and sustainability goals.
The company had set a significant target to reduce its municipal waste by 50% by the year 2025, underscoring a commitment to minimizing its environmental footprint. Achieving such a goal would require substantial improvements in waste reduction, reuse, and recycling strategies across all its business units.
Galenica's operational energy needs across its pharmacies, warehouses, and administrative offices directly influence its environmental impact. The company is actively addressing this by prioritizing energy efficiency measures and the adoption of renewable energy sources.
A significant commitment is Galenica's objective to procure 100% of its electricity from renewable sources for all its sites starting in 2025. This strategic shift aims to substantially reduce its carbon footprint and align with global sustainability trends in the healthcare and pharmaceutical sectors.
Sustainable Logistics and Transportation
Galenica's extensive logistics and transportation network is a key area impacting its environmental footprint. The company is focusing on reducing its reliance on fossil fuels, with a goal to incorporate more renewable energy sources into its vehicle fleet. For instance, by the end of 2024, Galenica aims to have 25% of its light commercial vehicle fleet powered by alternative fuels.
The joint venture Health Supply is instrumental in optimizing pharmaceutical logistics, specifically targeting the implementation of sustainable transport solutions. This venture is exploring electric vehicles and route optimization software to minimize emissions. By 2025, Health Supply anticipates a 15% reduction in CO2 emissions per kilometer driven through these initiatives.
- Fleet Transition: Galenica is progressively integrating low-emission vehicles, with electric and hybrid models making up 10% of its new vehicle acquisitions in 2024.
- Route Optimization: Advanced logistics software is being deployed to reduce mileage and fuel consumption, targeting a 5% decrease in fuel usage by the end of 2025.
- Partnership for Sustainability: Health Supply's focus on sustainable transport solutions is expected to set new industry benchmarks for environmental responsibility in pharmaceutical distribution.
- Carbon Footprint Reduction Targets: Galenica has set a company-wide goal to reduce its Scope 1 and Scope 2 emissions by 30% by 2030 compared to a 2022 baseline.
Eco-friendly Product Development and Packaging
Consumers are increasingly prioritizing eco-friendly products and sustainable packaging, with a significant portion willing to pay more for them. For Galenica, this translates to a need to scrutinize ingredient sourcing for its health and beauty lines, ensuring they align with sustainable practices. For example, studies in 2024 indicated that over 60% of consumers actively seek out brands with strong environmental commitments.
Regulatory bodies are also tightening their grip on environmental standards. This includes mandates for reduced plastic usage and increased recyclability in packaging. Galenica must adapt its packaging strategies to comply with these evolving regulations, which are projected to become even more stringent by 2025, impacting material choices and waste management processes.
The company's proactive approach to sustainability is also evident in its operational choices, such as designing eco-friendly exhibition booths. This commitment extends beyond product packaging, signaling a broader corporate responsibility towards environmental stewardship. In 2024, industry surveys showed that companies with visible sustainability initiatives often experience improved brand perception and customer loyalty.
Key considerations for Galenica include:
- Sustainable Ingredient Sourcing: Ensuring raw materials are ethically and environmentally sourced, potentially reducing Galenica's carbon footprint by 15% on key product lines by 2025.
- Eco-Conscious Packaging: Transitioning to recyclable, biodegradable, or compostable packaging materials, aiming to reduce packaging waste by 20% annually.
- Circular Economy Integration: Exploring packaging take-back programs or partnerships to promote reuse and recycling, aligning with global waste reduction targets.
- Supply Chain Transparency: Enhancing visibility into the environmental impact of its entire supply chain to identify and mitigate risks.
Galenica's operations are increasingly influenced by environmental factors, with new regulations from the EU and Switzerland set to tighten sustainability standards in 2025, particularly concerning eco-friendly drug production and waste management.
The company's commitment to reducing its environmental impact is demonstrated through its Environmental Code of Conduct and ambitious targets, such as achieving 100% renewable electricity procurement for all sites by 2025.
Consumer demand for eco-friendly products is rising, with over 60% of consumers in 2024 seeking brands with strong environmental commitments, pushing Galenica to focus on sustainable ingredient sourcing and packaging.
| Environmental Target | Current Status/Progress | Target Year |
| Reduce municipal waste by 50% | Ongoing initiatives | 2025 |
| Procure 100% renewable electricity | Targeted implementation | 2025 |
| 25% of light commercial vehicle fleet powered by alternative fuels | Aiming for achievement | End of 2024 |
| Reduce Scope 1 and Scope 2 emissions by 30% | Baseline established | 2030 (from 2022) |
PESTLE Analysis Data Sources
Our Galenica PESTLE Analysis is built on a robust foundation of data from leading pharmaceutical industry associations, regulatory bodies like the FDA and EMA, and reputable market research firms. We integrate economic indicators, scientific journals, and global health reports to ensure comprehensive insights.
