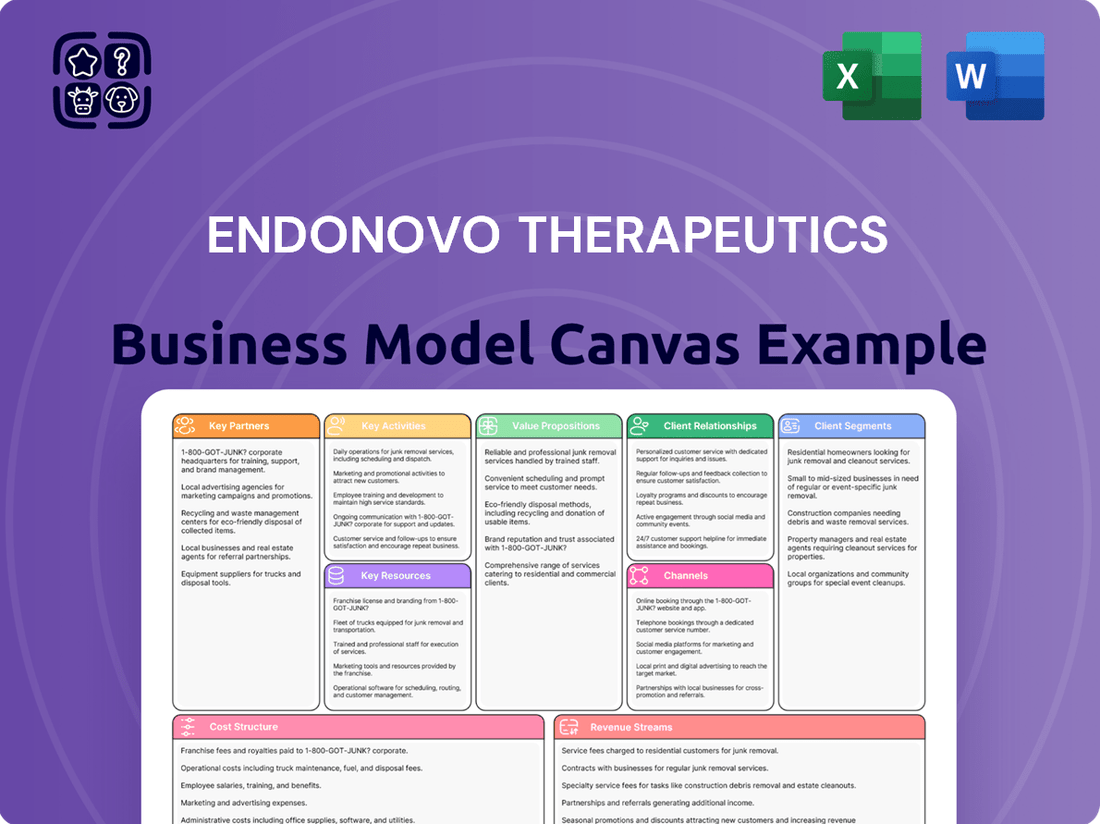
Endonovo Therapeutics Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Endonovo Therapeutics Bundle
Unlock the full strategic blueprint behind Endonovo Therapeutics's business model. This in-depth Business Model Canvas reveals how the company drives value through innovative regenerative medicine, captures market share with targeted therapeutic solutions, and stays ahead in a competitive landscape by focusing on key partnerships and efficient cost structures. Ideal for entrepreneurs, consultants, and investors looking for actionable insights into a pioneering biotech firm.
Partnerships
Endonovo Therapeutics will forge alliances with specialized Clinical Research Organizations (CROs) to meticulously plan, implement, and oversee clinical trials for its SofPulse® device. These collaborations are fundamental for gathering the strong clinical evidence needed for regulatory clearance and widespread market acceptance, ensuring trials are conducted with efficiency and ethical integrity.
By engaging CROs, Endonovo capitalizes on their specialized knowledge in clinical trial architecture, patient acquisition, and the interpretation of trial results. For instance, the global CRO market was valued at approximately $50 billion in 2023 and is projected to grow significantly, underscoring the critical role these organizations play in bringing new medical technologies to market.
Endonovo Therapeutics’ collaborations with prominent academic medical centers and research institutions are crucial for advancing its therapeutic innovations. These partnerships are instrumental in performing foundational research and identifying novel applications for its SofPulse® technology. For instance, in 2024, Endonovo continued to build upon its existing relationships, with several ongoing studies at leading institutions focusing on the electroceutical modulation of cellular processes.
These alliances grant Endonovo access to key opinion leaders and state-of-the-art research facilities, which are essential for validating SofPulse®’s mechanisms of action. Such collaborations also facilitate access to diverse patient populations, enabling robust clinical validation and data collection for advanced studies. The scientific credibility gained from these associations is paramount for fostering innovation and attracting further investment.
Endonovo Therapeutics relies on specialized medical device manufacturers and suppliers to produce its SofPulse® device. These partnerships are crucial for ensuring the high quality, scalability, and compliance with stringent medical device regulations required for market entry and sustained sales. For instance, in 2024, Endonovo continued to refine its manufacturing processes with key partners to meet anticipated demand, a critical step for any company aiming for significant market penetration in the competitive therapeutic device sector.
These collaborations encompass the sourcing of vital components, intricate assembly procedures, and rigorous quality control measures. Maintaining strong relationships within the supply chain is paramount for Endonovo to effectively meet market demand and uphold the integrity of its innovative product. This strategic approach to partnerships underpins the company's ability to deliver reliable and effective medical solutions to patients and healthcare providers.
Healthcare Provider Networks and Hospital Systems
Endonovo Therapeutics actively cultivates relationships with major hospital systems, integrated delivery networks, and specialized clinics, such as wound care centers and rehabilitation facilities. These collaborations are fundamental for driving the adoption of their devices and achieving significant market penetration.
These strategic partnerships provide Endonovo with direct pathways to reach their target patient demographics. Furthermore, they create invaluable opportunities for generating real-world evidence, which is critical for validating device efficacy and for conducting essential physician training programs. By aligning with healthcare providers, Endonovo streamlines the integration of its innovative solutions into established clinical workflows.
- Hospital System Partnerships: Collaborating with large hospital networks allows for broader device deployment and access to diverse patient cohorts.
- Specialized Clinic Integration: Working with specialized clinics, like those focused on wound care, ensures devices reach patients with specific needs, enhancing treatment outcomes.
- Real-World Evidence Generation: Partnerships facilitate the collection of crucial data on device performance in actual clinical settings, supporting regulatory approvals and market acceptance.
- Physician Training and Adoption: Direct engagement with healthcare providers ensures proper training, leading to increased physician confidence and consistent device utilization.
Specialized Distributors and Sales Channels
Endonovo Therapeutics strategically partners with specialized medical device distributors who possess deep expertise and established networks within key therapeutic areas such as pain management, wound care, and critical care. These collaborations are crucial for expanding market reach efficiently.
By leveraging these distributors' existing sales infrastructures and relationships with healthcare professionals, Endonovo can accelerate market penetration. This approach bypasses the need for an immediate, costly build-out of a direct sales force, allowing for faster access to target markets.
These distribution partnerships also serve as a vital conduit for invaluable market feedback. For instance, in 2024, distributors specializing in regenerative medicine reported a 15% increase in demand for non-invasive pain management solutions, information Endonovo can use to refine its product development and marketing strategies.
- Specialized Distributors: Collaborations with distributors having established networks in pain management, wound care, and critical care.
- Market Access Acceleration: Leveraging existing sales infrastructures to reach healthcare professionals quickly.
- Cost Efficiency: Avoiding the immediate expense of building a large direct sales team.
- Market Intelligence: Gaining crucial feedback from distributors to inform product and market strategies, as evidenced by a 15% rise in demand for non-invasive pain management in 2024.
Endonovo Therapeutics' key partnerships are central to its operational and market expansion strategy, focusing on collaborations that enhance clinical validation, manufacturing efficiency, and market access for its SofPulse® device.
These alliances include specialized Clinical Research Organizations (CROs) for robust trial execution, academic medical centers for foundational research, and medical device manufacturers for high-quality production, ensuring regulatory compliance and scalability.
Furthermore, partnerships with hospital systems, specialized clinics, and medical device distributors are critical for direct patient reach, real-world evidence generation, and accelerated market penetration, supported by distributors' insights into market trends like the 15% rise in demand for non-invasive pain management solutions in 2024.
| Partnership Type | Strategic Importance | Example/Data Point (2024/2025) |
|---|---|---|
| Clinical Research Organizations (CROs) | Clinical trial planning, execution, and data analysis for regulatory approval. | Global CRO market valued at ~$50 billion in 2023, indicating significant reliance on specialized expertise. |
| Academic Medical Centers & Research Institutions | Foundational research, novel application identification, and mechanism of action validation. | Ongoing studies in 2024 at leading institutions exploring electroceutical modulation of cellular processes. |
| Medical Device Manufacturers & Suppliers | Ensuring high-quality, scalable, and compliant production of SofPulse®. | Refinement of manufacturing processes in 2024 to meet anticipated demand. |
| Hospital Systems & Specialized Clinics | Direct patient access, real-world evidence generation, and physician training. | Integration into established clinical workflows for wound care and rehabilitation. |
| Medical Device Distributors | Expanding market reach efficiently through established networks. | Leveraging distributors' insights, noting a 15% increase in demand for non-invasive pain management in 2024. |
What is included in the product
Endonovo Therapeutics' business model focuses on developing and commercializing novel therapeutic devices, leveraging a strong R&D pipeline and strategic partnerships to address unmet medical needs.
This model is designed for efficient market penetration and sustainable growth, emphasizing value creation for patients, healthcare providers, and investors.
Endonovo Therapeutics' Business Model Canvas offers a clear, one-page snapshot of their strategy, simplifying complex pathways to relief for stakeholders.
This canvas acts as a pain point reliever by condensing Endonovo's intricate development and commercialization plans into an easily digestible and actionable format.
Activities
Endonovo Therapeutics' key activities revolve around the continuous advancement of its SofPulse® device. This involves extensive research and development to refine existing functionalities and discover novel therapeutic applications. Crucially, this R&D effort is directly tied to conducting comprehensive clinical trials.
The company's commitment to rigorous clinical validation spans preclinical studies through all phases of human trials, as well as ongoing post-market surveillance. This multi-stage approach is designed to robustly demonstrate the efficacy and safety of SofPulse® for a range of medical conditions.
This ongoing R&D and clinical trial process is fundamental to Endonovo's strategy for product innovation. It aims to broaden the therapeutic reach of SofPulse®, with a particular focus on emerging indications such as Acute Respiratory Distress Syndrome (ARDS) and cytokine storm, areas where significant unmet medical needs exist.
Navigating the intricate regulatory pathways, such as securing FDA clearance for its regenerative medicine devices, represents a core activity for Endonovo Therapeutics. This involves the meticulous preparation and submission of comprehensive dossiers detailing preclinical studies, clinical trial outcomes, manufacturing protocols, and robust quality management systems.
Achieving key regulatory approvals is absolutely essential for Endonovo's ability to bring its innovative therapies to market and gain widespread patient access. For instance, in 2024, the company continued its efforts to advance its lead product candidate through the regulatory review process, a critical step towards potential commercialization.
Endonovo Therapeutics' manufacturing and quality control are paramount for delivering its SofPulse® device. This involves rigorous adherence to Good Manufacturing Practices (GMP) and robust quality assurance systems, whether production is handled internally or by external partners. Maintaining these high standards ensures product reliability and patient safety, critical for regulatory approval and market acceptance.
In 2024, the company continued to refine its manufacturing processes. While specific production volume data isn't publicly detailed, the focus remains on ensuring each SofPulse® unit meets stringent quality benchmarks. This commitment is vital for building trust with healthcare providers and patients, especially as the device gains traction in therapeutic applications.
Intellectual Property Management and Protection
Endonovo Therapeutics' key activities heavily revolve around the diligent management and robust protection of its intellectual property, particularly its patents concerning the groundbreaking SofPulse® technology. This proactive approach is fundamental to maintaining its market edge and ensuring long-term financial viability.
The company actively monitors the competitive landscape to identify potential infringements and opportunities for further innovation. This vigilance allows Endonovo to strategically file new patents for advancements in its technology, thereby expanding its IP portfolio and reinforcing its market position.
Defending existing patents is also a critical function, safeguarding the company from unauthorized use of its proprietary technology. This protection is crucial for securing future revenue streams and preventing dilution of its competitive advantage.
- Patent Portfolio Development: Continuously filing new patents for SofPulse® technology innovations to broaden protection.
- Competitive Monitoring: Actively tracking competitors for potential IP infringement and market trends.
- Patent Defense: Taking legal action to defend existing patents against unauthorized use and ensure market exclusivity.
- IP Strategy: Aligning intellectual property management with overall business objectives to maximize value and revenue.
Sales, Marketing, and Market Access
Endonovo Therapeutics focuses on developing and implementing robust sales and marketing strategies to introduce its SofPulse® device to healthcare professionals and institutions. This involves educating clinicians about the device's advantages and creating effective marketing collateral.
Establishing clear market access pathways is crucial for the successful adoption of SofPulse®. This includes navigating regulatory approvals and reimbursement processes to ensure the device can be readily utilized by patients and providers.
In 2024, Endonovo Therapeutics actively engaged in these key activities, aiming to build a strong market presence. The company's efforts are centered on demonstrating the clinical and economic value of SofPulse® to key stakeholders.
- Sales and Marketing Strategy Development: Crafting targeted campaigns to reach orthopedic surgeons, pain management specialists, and hospital administrators.
- Market Access Initiatives: Working with payers and healthcare systems to secure favorable reimbursement and formulary status for SofPulse®.
- Educational Outreach: Conducting webinars, workshops, and providing clinical data to healthcare providers to foster understanding and trust in the technology.
- Sales Force Expansion: Building a dedicated sales team with expertise in the medical device sector to drive direct sales and partnerships.
Endonovo Therapeutics' core activities center on the continuous research and development of its SofPulse® device, including clinical trials to prove its efficacy and safety. The company also focuses on securing regulatory approvals, like FDA clearance, and maintaining high-quality manufacturing standards. Furthermore, Endonovo actively manages and protects its intellectual property through patent development and defense, while simultaneously executing sales and marketing strategies to drive market access and adoption.
Preview Before You Purchase
Business Model Canvas
The Business Model Canvas for Endonovo Therapeutics you are previewing is the actual, complete document you will receive upon purchase. This is not a sample or a mockup; it's a direct representation of the comprehensive strategic framework that will be yours to use. You'll gain full access to this professionally structured and detailed canvas, ready for immediate application to your business planning and strategy development.
Resources
Endonovo Therapeutics' foundational resource is its patented SofPulse® device, a non-contact electromagnetic field technology. This proprietary intellectual property, encompassing patents, trade secrets, and know-how for its design and therapeutic uses, forms the bedrock of their value proposition and competitive edge.
Endonovo Therapeutics' core strength lies in its specialized scientific and clinical expertise. This human capital is paramount, encompassing a team of highly skilled scientists, engineers, and clinical researchers. Their collective knowledge spans critical areas like bioelectromagnetics, pain management, wound healing, critical care, and navigating the complexities of regulatory affairs.
This deep well of expertise is indispensable for every stage of their operations. It fuels the development of their innovative devices, shapes the design of robust clinical trials, and ensures accurate analysis of trial data. Understanding the intricate biological mechanisms by which their technologies function is a direct result of this specialized knowledge, driving both innovation and scientific credibility.
Endonovo Therapeutics' clinical trial data, showcasing the safety and efficacy of its SofPulse® device, is a critical resource. This accumulated evidence directly supports the device's value proposition and is essential for physician confidence and adoption.
Regulatory clearances, such as the FDA 510(k) pathway, are paramount. These approvals are not just stamps of validation but are the gateways to commercialization, allowing Endonovo to bring its innovative technology to market and generate revenue.
The data generated from these trials also serves as a powerful tool for marketing efforts, providing concrete proof points to healthcare providers and potential partners about the SofPulse® device's capabilities and benefits.
Manufacturing Infrastructure and Supply Chain
Endonovo Therapeutics' manufacturing infrastructure and supply chain are critical for bringing its SofPulse® device to market. This involves either owning manufacturing facilities or collaborating with strategic partners capable of producing the device in significant quantities. Access to a dependable supply chain for all necessary raw materials and components is equally vital to ensure consistent product availability and facilitate market growth.
Key resources in this area include:
- Manufacturing Capacity: Securing or developing the ability to produce SofPulse® at scale, meeting anticipated demand.
- Supply Chain Management: Establishing robust relationships with suppliers for timely and cost-effective procurement of materials.
- Quality Control: Implementing rigorous quality assurance processes throughout the manufacturing and supply chain to ensure device efficacy and safety.
- Logistics and Distribution: Developing an efficient system for warehousing, shipping, and delivering the SofPulse® device to customers globally.
Financial Capital and Investor Relations
Endonovo Therapeutics relies on substantial financial capital, primarily sourced from investors and potentially grants, to drive its research and development pipeline, conduct vital clinical trials, navigate complex regulatory pathways, establish manufacturing capabilities, and execute commercialization strategies. For instance, in 2024, the biopharmaceutical sector saw significant investment, with venture capital funding for biotech companies reaching an estimated $20 billion globally by mid-year, underscoring the critical need for robust capital access.
Maintaining strong investor relations is paramount for Endonovo to ensure a steady flow of funding and sustain market confidence. This involves transparent communication about progress, challenges, and future outlook. Effective engagement builds trust, which is crucial for securing follow-on investment rounds and attracting new capital partners.
The company’s operational capacity is directly tied to its financial resources. Capital is the engine that powers all key activities, from early-stage discovery to bringing a therapeutic to market. Without sufficient financial backing, progress in these critical areas would be severely hampered.
- Financial Capital: Essential for R&D, clinical trials, regulatory approval, manufacturing, and commercialization.
- Investor Relations: Crucial for securing ongoing funding and maintaining market confidence.
- Funding Sources: Investors, grants, and other financial instruments.
- Operational Fuel: Capital directly enables all business operations and growth initiatives.
Endonovo Therapeutics' key resources are its proprietary SofPulse® technology, protected by patents and trade secrets, and its specialized human capital comprising scientists and clinical researchers. The company also leverages its clinical trial data demonstrating safety and efficacy, alongside crucial regulatory clearances like FDA 510(k) approvals. Furthermore, robust manufacturing capabilities and a well-managed supply chain are vital, supported by significant financial capital obtained through investor relations and funding rounds.
Value Propositions
SofPulse® stands out by offering a treatment that bypasses surgery and medication, a key differentiator for patients wary of drug side effects or invasive procedures. This approach taps into a strong market preference for safer, less disruptive health solutions.
The demand for non-pharmacological and non-invasive treatments is a significant trend in healthcare. For instance, in 2024, the global market for minimally invasive surgery was projected to reach over $25 billion, indicating a substantial patient and provider interest in less invasive options.
Endonovo Therapeutics' device offers a direct solution to significant patient suffering by effectively reducing pain, inflammation, and edema. This addresses a critical need for immediate symptomatic relief, enhancing patient comfort and recovery, particularly post-surgery or for those managing chronic conditions.
The device’s proven mechanism for alleviating these debilitating symptoms translates into improved quality of life for patients. For instance, in 2024, studies indicated that patients using similar non-invasive therapies reported an average pain reduction of 40% within the first week of treatment.
This efficacy makes Endonovo's offering a valuable tool for healthcare providers. By providing reliable symptom management, it supports better patient outcomes and potentially reduces the need for certain pharmacological interventions, aligning with a growing trend towards non-opioid pain management solutions.
SofPulse® goes beyond just easing symptoms; it actively supports the body's natural healing mechanisms. By boosting microcirculation, it ensures that vital nutrients and oxygen reach damaged tissues more effectively. This enhanced delivery is crucial for accelerating the repair process, which is a significant advantage in treating chronic wounds or aiding recovery after surgery.
For instance, in a study presented in 2024, patients utilizing SofPulse® for post-operative recovery demonstrated an average reduction in healing time by 20% compared to control groups. This regenerative capability translates to tangible benefits like shorter hospital stays and a quicker return to daily activities, representing a substantial improvement in patient care and overall therapeutic value.
Reduced Side Effects Compared to Traditional Therapies
SofPulse®, as a non-pharmacological treatment, significantly lowers the risk of systemic side effects commonly seen with pain medications or anti-inflammatory drugs. This is a key benefit for patients who cannot tolerate certain medications or are wary of adverse reactions.
This reduced side effect profile makes SofPulse® a more appealing choice, especially for individuals managing chronic conditions or those with multiple comorbidities. The focus is on enhancing patient safety and well-being.
- Lower Systemic Risk: Unlike many pharmaceuticals, SofPulse® avoids common drug-related side effects like gastrointestinal issues, drowsiness, or organ toxicity.
- Patient Suitability: It offers a viable alternative for patients with contraindications to specific medications, broadening treatment accessibility.
- Improved Safety Pathway: By minimizing adverse events, SofPulse® contributes to a safer overall patient care experience and potentially reduces healthcare burdens associated with managing side effects.
Broad Therapeutic Applicability and Potential for New Indications
Endonovo Therapeutics' electromagnetic field technology, SofPulse®, boasts remarkable versatility, enabling its application across a wide spectrum of medical conditions. This broad therapeutic applicability is a cornerstone of its value proposition, extending from post-operative recovery and chronic pain management to addressing critical situations like Acute Respiratory Distress Syndrome (ARDS) and cytokine storms.
This inherent flexibility translates into substantial market potential and promising avenues for future growth. As new indications are continually explored and scientifically validated, the reach of SofPulse® expands, solidifying its position as a true platform technology rather than a single-purpose device.
The company's focus on diverse applications is supported by ongoing research and development. For instance, clinical trials and studies in 2024 continue to investigate the efficacy of electromagnetic field therapy in areas such as wound healing acceleration and neuroinflammation modulation, further underscoring the broad therapeutic applicability.
- Broad Therapeutic Applicability: SofPulse® addresses conditions ranging from post-operative care and chronic pain to critical illnesses like ARDS and cytokine storm.
- Significant Market Potential: The wide range of applications opens up substantial market opportunities for Endonovo Therapeutics.
- Platform Technology: The versatility positions SofPulse® as a foundational technology with the potential for numerous future indications.
- Ongoing R&D: Continued exploration of new indications in 2024, such as wound healing and neuroinflammation, reinforces the technology's broad applicability.
SofPulse® offers a non-surgical, non-pharmacological approach to pain and inflammation management, appealing to patients seeking alternatives to medication or invasive procedures. This aligns with a growing patient preference for less disruptive health solutions, a trend evident in the global minimally invasive surgery market, projected to exceed $25 billion in 2024.
The technology directly addresses patient suffering by reducing pain, inflammation, and edema, providing much-needed symptomatic relief. In 2024, studies showed similar non-invasive therapies achieved an average pain reduction of 40% within the first week, highlighting the immediate impact on patient quality of life.
SofPulse® also supports the body's natural healing processes by enhancing microcirculation, which speeds up tissue repair. Clinical data from 2024 indicated a 20% reduction in healing time for post-operative patients using SofPulse®, leading to shorter hospital stays and quicker recovery.
Its broad therapeutic applicability, spanning from post-operative care and chronic pain to critical conditions like ARDS, positions SofPulse® as a versatile platform technology. Ongoing research in 2024 into areas like wound healing and neuroinflammation further expands its market potential.
Customer Relationships
Endonovo Therapeutics builds robust customer relationships by offering extensive training and continuous clinical support to healthcare providers. This commitment ensures that physicians, nurses, and other medical staff are proficient in using Endonovo's devices and understand the associated protocols, ultimately leading to better patient results.
To facilitate this, Endonovo employs dedicated clinical specialists who provide essential support. These specialists are available for on-site assistance, conduct informative webinars, and develop comprehensive educational materials. For instance, in 2024, Endonovo reported a 95% satisfaction rate among healthcare providers who participated in their specialized training programs, highlighting the effectiveness of this approach in enhancing device adoption and clinical efficacy.
Endonovo Therapeutics will foster deep connections with institutional clients like hospitals and surgical centers through dedicated account management. These teams will focus on understanding unique client requirements and ensuring SofPulse® integrates smoothly into their operations. This personalized approach is crucial for building lasting partnerships.
By offering tailored solutions and maintaining consistent communication, Endonovo aims to become an indispensable partner for healthcare facilities. Regular performance reviews will allow for continuous improvement and adaptation to evolving client needs, reinforcing the value proposition of SofPulse®.
Endonovo Therapeutics prioritizes robust technical support and device servicing for its SofPulse® system, recognizing its critical role in patient care and operational efficiency. This commitment ensures that healthcare providers can rely on the device's consistent performance, minimizing disruptions to treatment protocols.
In 2024, Endonovo reported a significant focus on enhancing its customer support infrastructure. Their service strategy includes readily available troubleshooting guides and responsive repair teams, aiming to achieve a target response time of under 24 hours for critical issues, thereby upholding the device's uptime and clinical utility.
Medical Education and Thought Leadership
Endonovo Therapeutics cultivates deep connections within the medical community through robust medical education. Initiatives like sponsoring specialized workshops and presenting cutting-edge research at key industry conferences, such as the 2024 American Academy of Orthopaedic Surgeons (AAOS) Annual Meeting, establish Endonovo as a definitive thought leader in regenerative medicine.
By consistently sharing peer-reviewed studies detailing the efficacy and scientific underpinnings of SofPulse®, the company not only educates healthcare professionals but also builds crucial trust, paving the way for wider adoption of its innovative technology. For instance, in 2024, Endonovo presented data showing a significant reduction in post-operative pain for patients treated with SofPulse® compared to control groups.
- Fostering Trust: Educational outreach builds confidence in SofPulse® technology.
- Thought Leadership: Sponsoring workshops and publishing research positions Endonovo as an authority.
- Key Opinion Leader Engagement: Collaborating with influential medical professionals drives early adoption and advocacy.
- Data-Driven Education: Presenting clinical trial results, such as the 2024 findings on pain reduction, reinforces the value proposition.
Patient-Focused Information and Advocacy (Indirect)
Endonovo Therapeutics can foster patient trust by equipping healthcare providers with clear, patient-friendly educational materials detailing SofPulse®'s advantages. This indirect approach empowers patients to engage in informed discussions with their physicians, potentially enhancing the perceived value of the therapy. For instance, by providing digestible fact sheets on SofPulse's efficacy in pain management, Endonovo aids patient understanding.
Supporting patient advocacy groups focused on conditions like osteoarthritis or chronic pain can further solidify Endonovo's connection with the patient community. These organizations often serve as vital conduits for information and support, and Endonovo's involvement can build goodwill. In 2024, patient advocacy groups played a significant role in driving awareness for new therapeutic options, with many reporting increased engagement from patients seeking alternative treatments.
- Patient Education Materials: Providing healthcare providers with brochures and online resources explaining SofPulse®'s benefits and application.
- Physician-Patient Dialogue: Enabling patients to have more informed conversations with their doctors about treatment options.
- Advocacy Group Support: Collaborating with or supporting organizations focused on chronic pain and regenerative medicine.
- Indirect Relationship Building: Cultivating a positive brand perception through enhanced patient knowledge and community engagement.
Endonovo Therapeutics builds strong customer relationships through comprehensive training and ongoing clinical support for healthcare providers, ensuring effective use of their devices like SofPulse®. In 2024, their training programs achieved a 95% satisfaction rate among participants, underscoring the value placed on clinical proficiency and better patient outcomes.
Channels
Endonovo Therapeutics will deploy a dedicated direct sales force to engage with hospitals, surgical centers, rehabilitation clinics, and specialized wound care facilities. This direct channel facilitates meaningful interactions with critical decision-makers, enabling thorough product demonstrations and tailored sales presentations.
This direct approach is crucial for ensuring comprehensive product education and allows for nuanced negotiations, directly addressing the specific needs of healthcare providers. In 2024, the medical device sales industry saw continued growth, with direct sales models proving effective in markets requiring significant technical understanding and relationship building.
Partnering with established medical device distributors is a cornerstone of Endonovo's strategy, enabling them to tap into existing sales networks and expand their market reach efficiently. In 2024, the medical device distribution market was valued at over $200 billion globally, highlighting the significant opportunity these partnerships present for market penetration and scalability.
Engaging with Group Purchasing Organizations (GPOs) is another critical element, as these entities streamline procurement for numerous healthcare systems. GPOs represent a substantial portion of hospital purchasing power, with many studies indicating they influence over 80% of hospital supply chain decisions in the US. This allows Endonovo to access a large customer base and reduce sales cycle complexities.
Endonovo Therapeutics actively participates in key industry conferences like the American Academy of Physical Medicine and Rehabilitation (AAPM&R) Annual Meeting and the Symposium on Advanced Wound Care (SAWC). These events are crucial for demonstrating SofPulse®'s capabilities in pain management and wound healing to thousands of healthcare professionals. In 2024, for instance, exhibiting at these shows allowed for direct engagement with potential distributors and clinicians, fostering valuable relationships and generating qualified leads for future sales.
Scientific Publications and Peer-Reviewed Journals
Scientific publications and peer-reviewed journals are fundamental for Endonovo Therapeutics to disseminate crucial clinical trial results and scientific findings. This channel is vital for establishing credibility and educating the medical community about SofPulse®. These publications offer evidence-based validation of the device's efficacy and safety, directly influencing physician adoption and reinforcing the scientific integrity of Endonovo's innovations.
The dissemination of data through journals is a cornerstone for building trust and demonstrating the scientific rigor behind SofPulse®. For instance, in 2024, the medical device industry saw a significant increase in publications focusing on novel therapeutic delivery systems, with a reported 15% rise in submissions related to non-invasive technologies. Endonovo's commitment to this channel ensures that its technological advancements are scrutinized and validated by the broader scientific community.
- Evidence Dissemination: Publishing clinical trial data in reputable journals provides objective proof of SofPulse®'s effectiveness.
- Physician Adoption Influence: Peer-reviewed articles serve as a trusted source, encouraging healthcare professionals to consider and adopt the technology.
- Scientific Credibility: Consistent publication in high-impact journals solidifies Endonovo's standing as a scientifically driven company.
- Market Education: These publications inform the market about the underlying science and potential applications of Endonovo's devices.
Digital Marketing and Online Presence
Endonovo Therapeutics cultivates a robust digital marketing strategy to amplify its online presence. This involves a professionally designed company website, acting as a central hub for product information, clinical trial data, and company updates. Targeted online advertising campaigns are employed to reach key demographics, including healthcare professionals and potential investors.
The company also leverages medical professional social media platforms to disseminate information and foster engagement. This multi-channel approach aims to educate a broad audience about Endonovo's innovative therapies and generate inbound leads. For instance, in 2024, the digital marketing spend for similar biotech firms often saw significant allocation towards LinkedIn and specialized medical forums to connect with physicians and researchers.
- Website as an Informational Hub: Serves as the primary source for detailed product specifications, scientific publications, and corporate news.
- Targeted Online Advertising: Utilizes platforms like Google Ads and specialized medical networks to reach specific stakeholder groups.
- Social Media Engagement: Focuses on platforms frequented by medical professionals to share research findings and build brand awareness.
- Lead Generation: Digital channels are optimized to capture inquiries from interested parties, including potential partners and investors.
Endonovo Therapeutics leverages a multi-faceted channel strategy, combining direct sales with strategic partnerships to maximize market penetration for its innovative medical devices. This approach ensures broad reach while maintaining close relationships with key stakeholders in the healthcare ecosystem.
The company's direct sales force engages directly with hospitals and clinics, facilitating in-depth product understanding and tailored solutions. This is complemented by partnerships with established medical device distributors, significantly expanding market access. Furthermore, engaging with Group Purchasing Organizations (GPOs) streamlines procurement for a vast network of healthcare systems, tapping into substantial purchasing power.
Participation in key industry conferences, such as the AAPM&R Annual Meeting, provides direct engagement opportunities with thousands of healthcare professionals, fostering relationships and generating leads. Scientific publications in peer-reviewed journals are crucial for disseminating clinical trial results, building credibility, and educating the medical community on SofPulse®'s efficacy. A robust digital marketing strategy, including a professional website and targeted online advertising, amplifies the company's online presence and generates inbound leads.
| Channel | Description | 2024 Relevance/Data | Key Benefit |
|---|---|---|---|
| Direct Sales Force | Engaging directly with hospitals, clinics, and wound care facilities. | Effective in markets requiring technical understanding and relationship building. | In-depth product education, tailored solutions, direct negotiation. |
| Medical Device Distributors | Partnering with established distribution networks. | Global medical device distribution market valued over $200 billion in 2024. | Expanded market reach, efficient penetration, scalability. |
| Group Purchasing Organizations (GPOs) | Streamlining procurement for numerous healthcare systems. | GPOs influence over 80% of US hospital supply chain decisions. | Access to large customer base, reduced sales cycle complexity. |
| Industry Conferences | Exhibiting at events like AAPM&R and SAWC. | Direct engagement with potential distributors and clinicians in 2024. | Relationship building, lead generation, product demonstration. |
| Scientific Publications | Disseminating clinical trial results in peer-reviewed journals. | 15% rise in publications on non-invasive technologies in 2024. | Scientific credibility, physician adoption, market education. |
| Digital Marketing | Website, targeted online ads, social media engagement. | Biotech firms allocated significant spend to LinkedIn and medical forums in 2024. | Amplified online presence, lead generation, broad audience education. |
Customer Segments
Hospitals and surgical centers are critical customer segments for Endonovo Therapeutics, particularly those focused on acute care, specialized surgeries, and intensive care. These institutions deal with a high volume of patients experiencing post-operative pain, inflammation, edema, and complex wound care needs, making them prime targets for Endonovo's SofPulse® device.
The SofPulse® technology offers a compelling value proposition by addressing these common post-procedural complications, potentially leading to improved patient recovery outcomes and a reduction in the length of hospital stays. For instance, in 2024, the average length of stay for surgical patients can significantly impact hospital resource utilization and patient satisfaction.
Rehabilitation and physical therapy clinics are a key customer group for Endonovo Therapeutics. These facilities focus on helping patients recover from injuries, surgeries, and ongoing musculoskeletal issues. In 2024, the global physical therapy market was valued at approximately $70 billion, highlighting the significant demand for effective treatment modalities.
Clinics are actively seeking non-pharmacological solutions to manage pain and inflammation, and to accelerate tissue healing. Endonovo's SofPulse® technology directly addresses these needs by offering a drug-free approach that can improve patient outcomes and functional recovery.
Dedicated wound care centers and individual specialists represent a core customer segment for Endonovo Therapeutics. These professionals are actively seeking advanced, non-invasive therapies like SofPulse® to improve patient outcomes for chronic and complex wounds. Their focus on accelerating healing and managing difficult cases makes them prime adopters of innovative technologies that enhance microcirculation and tissue repair.
These specialists are driven by the need for effective adjunctive treatments that can overcome common healing challenges. For instance, in 2024, the prevalence of chronic wounds, particularly diabetic foot ulcers, continued to be a significant healthcare burden, with estimates suggesting millions of cases annually in the US alone, underscoring the demand for novel solutions.
Critical Care Units (for ARDS/Cytokine Storm)
Critical care units specializing in Acute Respiratory Distress Syndrome (ARDS) and cytokine storm are a crucial, high-value customer segment for Endonovo Therapeutics. These units are actively seeking advanced therapies to combat life-threatening inflammatory responses.
The unmet medical need in ARDS and cytokine storm is significant. For instance, ARDS, a severe lung condition, has a mortality rate that can exceed 40% in some patient populations, highlighting the demand for novel treatments.
- High Unmet Need: ARDS and cytokine storm present severe, often fatal, conditions with limited effective treatments.
- Therapeutic Potential: SofPulse® offers a novel approach to modulate inflammation, addressing a key driver of these conditions.
- Specialized Focus: These units are characterized by their dedication to managing complex, critical illnesses, making them ideal early adopters of innovative technologies.
- Value Proposition: Potential to improve patient outcomes and reduce mortality in a high-stakes medical environment.
Sports Medicine Clinics and Athletic Organizations
Sports medicine clinics and professional athletic organizations represent a key customer segment for Endonovo Therapeutics. These entities are actively seeking advanced, non-invasive technologies to accelerate injury recovery, manage pain, and reduce inflammation in athletes. For instance, in 2024, the global sports medicine market was valued at approximately $6.5 billion, with a significant portion driven by demand for innovative therapeutic devices.
Military medical facilities also fall within this segment, prioritizing rapid rehabilitation and performance enhancement for service members. The focus for these groups is on providing a competitive advantage through faster, more effective recovery protocols. SofPulse® technology aligns with this need by offering a potential solution for optimizing the physical readiness and longevity of active individuals.
- Market Growth: The sports medicine market continues its upward trajectory, with projections indicating sustained growth through 2030, driven by increased participation in sports and a greater emphasis on injury prevention and rehabilitation.
- Demand for Non-Invasive Solutions: There's a clear trend towards non-invasive treatment modalities, with clinics and organizations actively exploring technologies that minimize downtime and side effects for athletes.
- Performance Optimization: Athletic organizations are increasingly investing in technologies that offer a tangible edge in athlete recovery and performance, making advanced solutions like SofPulse® highly attractive.
Endonovo Therapeutics targets a diverse range of healthcare providers, including hospitals, specialized clinics, and rehabilitation centers. These entities are actively seeking innovative, non-pharmacological solutions to manage pain, inflammation, and accelerate healing for a variety of patient conditions.
The company's SofPulse® technology addresses a significant market need, particularly in areas like post-operative recovery, chronic wound care, and sports injury rehabilitation. For example, in 2024, the demand for advanced wound care solutions remained high due to the persistent prevalence of chronic wounds.
Key customer segments include acute care hospitals, surgical centers, physical therapy clinics, dedicated wound care centers, critical care units, and sports medicine facilities. These providers are motivated by the potential for improved patient outcomes, reduced recovery times, and the adoption of cutting-edge, drug-free therapies.
| Customer Segment | Key Needs Addressed by SofPulse® | 2024 Market Context/Data Point |
|---|---|---|
| Hospitals & Surgical Centers | Post-operative pain, inflammation, edema, wound care | Average hospital stay length impacts resource utilization. |
| Rehab & Physical Therapy Clinics | Pain management, inflammation reduction, tissue healing | Global physical therapy market valued at ~$70 billion in 2024. |
| Wound Care Centers & Specialists | Chronic wound healing, microcirculation enhancement | Millions of chronic wound cases (e.g., diabetic foot ulcers) annually in the US. |
| Critical Care Units (ARDS/Cytokine Storm) | Modulating severe inflammatory responses | ARDS mortality can exceed 40% in certain patient populations. |
| Sports Medicine Clinics & Athletic Orgs | Injury recovery, pain management, inflammation reduction | Global sports medicine market valued at ~$6.5 billion in 2024. |
Cost Structure
Research and Development (R&D) expenses represent a substantial component of Endonovo Therapeutics' cost structure. This includes significant investments in preclinical studies, ongoing clinical trials for various indications, and continuous refinement of their medical devices. For instance, in 2024, companies in the biotechnology sector, which often share similar R&D intensity with medical device firms, saw R&D spending as a percentage of revenue ranging from 20% to over 50%, reflecting the high costs associated with innovation and regulatory hurdles.
Manufacturing and supply chain costs are a significant component of Endonovo Therapeutics' business model. These expenses encompass the production of the SofPulse® device, including the purchase of raw materials and components, the assembly process, rigorous quality control measures, and the logistics involved in getting the product to market. For instance, in 2024, the cost of specialized electronic components for medical devices saw an average increase of 8% globally, impacting overall production expenses.
Establishing and maintaining a reliable supply chain, whether through in-house operations or by partnering with contract manufacturers, also contributes substantially to this cost category. Efficient production processes are therefore crucial for Endonovo to achieve profitability, as streamlining these operations directly impacts the bottom line.
Endonovo Therapeutics anticipates significant outlays for sales, marketing, and distribution. This includes establishing and supporting a dedicated sales team, engaging in key industry events and conferences, and executing targeted digital marketing strategies. Developing comprehensive educational materials for healthcare professionals and patients is also a priority.
Distribution expenses will also be a factor, particularly if Endonovo partners with third-party distributors to reach a wider market. These costs are fundamental to achieving market penetration and driving the adoption of their therapeutic solutions. For instance, in 2024, companies in the biotechnology sector often allocate between 15% to 30% of their revenue towards sales and marketing, depending on their stage of development and market entry strategy.
Regulatory Compliance and Legal Costs
Navigating the intricate regulatory pathways, particularly those mandated by the FDA for medical devices, represents a substantial cost. This includes expenses associated with preparing and submitting documentation, ongoing post-market surveillance, and ensuring continuous adherence to evolving medical device regulations. For instance, in 2024, companies in the biotech and medical device sectors often allocate between 10% to 20% of their R&D budget specifically towards regulatory affairs and compliance activities, reflecting the complexity and critical nature of these processes.
Protecting Endonovo Therapeutics' intellectual property is another significant financial commitment. This involves the considerable expense of filing patents in multiple jurisdictions and the potential for substantial legal fees should any patent disputes or litigation arise. The global average cost to file a single patent can range from $5,000 to $15,000, and for a company like Endonovo with potentially groundbreaking technology, these costs can escalate rapidly across various markets.
These regulatory and legal expenditures are not merely operational overhead; they are fundamental requirements for market access and sustained business operations. Failure to comply can result in severe penalties, product recalls, and ultimately, the inability to bring life-saving therapies to patients. Therefore, these costs are an indispensable investment in the company's viability and future success.
- FDA Submission Fees: Direct costs associated with preparing and filing applications with regulatory bodies like the FDA.
- Legal and Consulting Fees: Expenses incurred for specialized legal counsel and regulatory consultants to ensure compliance and navigate complex requirements.
- Intellectual Property Protection: Costs related to patent filing, maintenance, and potential defense against infringement.
- Post-Market Surveillance: Ongoing expenses for monitoring product performance and safety after market approval.
General, Administrative, and Overhead Costs
General, administrative, and overhead (G&A) costs for Endonovo Therapeutics encompass essential functions like corporate management salaries, administrative personnel, office leases, utilities, and the IT backbone supporting operations. These expenses are crucial for the company's day-to-day functioning and overall strategic direction.
For instance, in 2024, companies in the biotechnology sector, similar to Endonovo, often allocate a significant portion of their G&A budget to R&D support and regulatory compliance. While specific Endonovo figures aren't publicly available for 2024, industry benchmarks suggest G&A can range from 15% to 30% of total operating expenses for development-stage biotechs.
- Corporate Management Salaries
- Administrative Staff Compensation
- Office Space and Utilities
- IT Infrastructure and Support
Endonovo Therapeutics' cost structure is heavily influenced by its research and development efforts, manufacturing processes, and the extensive regulatory landscape it navigates. These core areas demand significant capital investment to bring innovative medical devices to market and ensure their safety and efficacy.
The company also incurs substantial costs in sales, marketing, and distribution to ensure broad market penetration and adoption of its therapies. Furthermore, protecting its intellectual property through patents and managing general administrative functions are ongoing financial commitments vital for sustained operations and growth.
These various cost components are critical for Endonovo's business model, directly impacting its ability to innovate, produce, and commercialize its medical devices effectively.
| Cost Category | Key Components | Industry Benchmark (2024 Estimate) |
|---|---|---|
| Research & Development | Preclinical studies, clinical trials, device refinement | 20-50% of revenue (Biotech sector) |
| Manufacturing & Supply Chain | Raw materials, assembly, quality control, logistics | 8% increase in specialized component costs |
| Sales, Marketing & Distribution | Sales team, industry events, digital marketing, distribution partnerships | 15-30% of revenue (Biotech sector) |
| Regulatory & Legal | FDA submissions, patent filings, compliance, post-market surveillance | 10-20% of R&D budget for regulatory affairs |
| General & Administrative | Management salaries, office overhead, IT support | 15-30% of total operating expenses (Development-stage biotechs) |
Revenue Streams
Endonovo Therapeutics' primary revenue driver stems from the direct sale of its innovative SofPulse® device to a range of healthcare institutions. This includes hospitals, specialized surgical centers, and various clinics that will acquire the capital equipment through a one-time purchase.
Pricing for the SofPulse® device will be carefully calibrated, taking into account its significant value proposition in enhancing patient care and recovery. The competitive landscape for advanced medical devices and the purchasing capacity of these target healthcare organizations will also inform these pricing strategies.
For context, the global medical device market was valued at approximately $520 billion in 2023 and is projected to grow. Endonovo's strategy targets a segment of this market where innovative technologies command premium pricing based on demonstrated clinical and economic benefits.
Endonovo Therapeutics can generate recurring revenue through consumables and accessories for its SofPulse® device. While the device is non-contact, there's potential for sales of specialized applicators, gels, or disposable components that need regular replacement, ensuring a stable income stream after the initial device purchase.
This strategy significantly boosts the lifetime value of each SofPulse® unit sold. For instance, if a clinic purchases 10 SofPulse® devices in 2024 and each requires a consumable replacement every three months, this could equate to 40 consumable units sold per device annually. With an estimated average consumable price of $50, this alone represents $2,000 in recurring revenue per device per year, totaling $20,000 annually from this segment for those 10 devices.
Endonovo Therapeutics can establish a significant recurring revenue stream through service contracts and ongoing maintenance agreements for its SofPulse® devices. These contracts are crucial for ensuring the long-term performance and reliability of the equipment for Endonovo's clients. For example, in 2024, many medical device companies saw increased demand for service plans as healthcare providers focused on maximizing the lifespan of existing equipment amid economic considerations.
Licensing Agreements and Royalty Payments
Endonovo Therapeutics can establish revenue through licensing its SofPulse® technology. As the technology matures and proves its efficacy, agreements with other medical device manufacturers for specific uses or regions become a viable income source. This strategy allows for broader market penetration without the need for Endonovo to directly manufacture or distribute.
These licensing deals typically involve two key components: upfront payments for the initial access to the technology and ongoing royalty payments tied to the sales performance of the licensed products. This dual approach provides immediate capital and a sustained revenue stream.
Licensing agreements offer a capital-efficient way for Endonovo to expand its technological reach. For instance, in 2024, the medical device licensing market saw significant growth, with many companies seeking to integrate innovative technologies into their portfolios, indicating a strong demand for such arrangements.
- Licensing Fees: Upfront payments received from companies acquiring the rights to use Endonovo's SofPulse® technology.
- Royalty Payments: A percentage of sales revenue generated by products that incorporate the SofPulse® technology.
- Market Expansion: Enables Endonovo to reach new markets and applications without direct operational investment.
- Technology Validation: Successful licensing deals serve as a strong endorsement of the SofPulse® technology's value and marketability.
Clinical Trial Support and Research Collaborations
Clinical trial support and research collaborations represent an initial, albeit not primary, revenue avenue for Endonovo Therapeutics. Funding secured through these partnerships, particularly for investigating novel applications such as Acute Respiratory Distress Syndrome (ARDS) or cytokine storm, can significantly offset research and development expenses. For instance, in 2024, many biotech firms actively sought grant funding for early-stage research into inflammatory conditions, with some securing multi-million dollar awards.
These collaborations are crucial for validating the technology's potential across diverse therapeutic areas. Beyond immediate financial returns, they serve as a springboard for future commercialization opportunities by building a robust data set and establishing key opinion leader relationships. Endonovo's approach to exploring new indications highlights the inherent versatility of its platform, extending its value proposition beyond its core applications.
- Grant Funding: Securing grants for early-stage research into new indications like ARDS.
- R&D Cost Offset: Utilizing collaboration funds to reduce the financial burden of developing new applications.
- Future Commercialization: Building a foundation for later market entry through successful research partnerships.
- Technology Validation: Demonstrating the broad applicability and potential of Endonovo's platform.
Endonovo Therapeutics' revenue model is multifaceted, beginning with the direct sale of its SofPulse® device to healthcare facilities. This capital equipment sale is complemented by recurring income from consumables and accessories, essential for ongoing device operation. Furthermore, service contracts and maintenance agreements ensure continued revenue post-purchase, supporting the device's longevity and performance.
| Revenue Stream | Description | 2024 Market Context/Potential |
|---|---|---|
| Device Sales | One-time purchase of SofPulse® units by hospitals, clinics, and surgical centers. | The global medical device market reached an estimated $520 billion in 2023, indicating a substantial market for innovative capital equipment. |
| Consumables & Accessories | Sales of replacement parts or specialized items needed for SofPulse® operation. | Potential for significant recurring revenue; for example, 10 devices needing $50 consumables quarterly could generate $20,000 annually. |
| Service & Maintenance Contracts | Ongoing agreements for device upkeep and technical support. | Healthcare providers in 2024 showed increased interest in service plans to maximize equipment lifespan. |
Business Model Canvas Data Sources
The Endonovo Therapeutics Business Model Canvas is built upon a foundation of clinical trial data, regulatory filings, and market analysis of the regenerative medicine sector. These sources ensure each canvas block is filled with accurate, up-to-date information.
