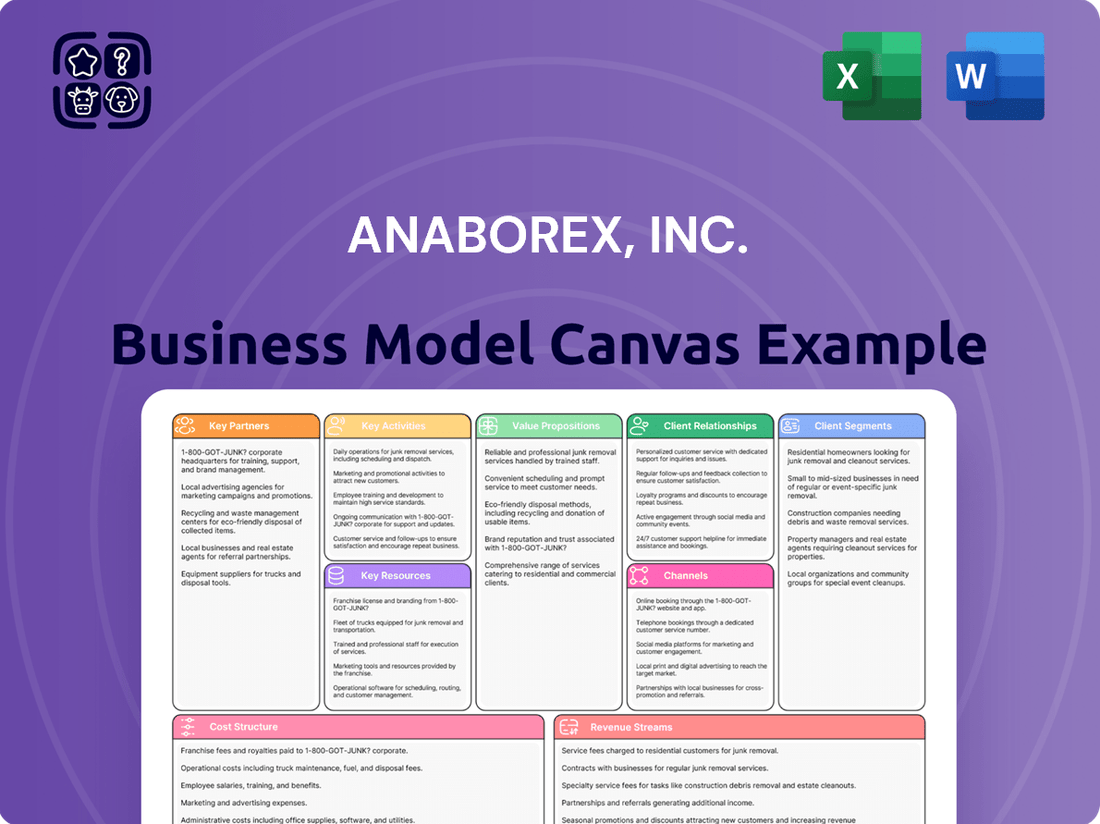
Anaborex, Inc. Business Model Canvas
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Anaborex, Inc. Bundle
Explore the core components of Anaborex, Inc.'s strategic framework with our comprehensive Business Model Canvas. This document details their key customer segments, unique value propositions, and revenue streams, offering a clear view of their operational engine. Ready to understand what truly drives Anaborex's success and how you can apply similar principles to your own ventures?
Partnerships
Anaborex, Inc. will forge co-development and licensing agreements with major pharmaceutical players. These collaborations are vital for shouldering the immense financial burden and inherent risks of late-stage clinical trials and bringing novel oncology and rare disease therapies to market.
These strategic alliances will grant Anaborex access to substantial resources, specialized knowledge, and established market channels. This significantly speeds up the process of getting life-changing treatments to patients who need them.
Anaborex, Inc. relies on Contract Research Organizations (CROs) to manage its clinical trials, ensuring both efficiency and adherence to regulations for its innovative therapies. These specialized partners are crucial for navigating the complexities of drug development, from initial trial design and patient enrollment to meticulous data handling and final regulatory submissions.
The global CRO market is experiencing robust growth, projected to reach approximately $95 billion by 2027, highlighting the increasing demand for their specialized expertise. This expansion is fueled by the rising complexity of clinical trials, particularly those focused on personalized medicine and novel therapeutic approaches, areas where Anaborex operates.
Anaborex, Inc. actively collaborates with top-tier academic and research institutions to drive groundbreaking scientific discoveries and advance preclinical development. These strategic alliances are crucial for accessing specialized research infrastructure, state-of-the-art technologies, and a continuous influx of brilliant scientific minds.
For instance, Anaborex's partnership with the University of California, San Francisco's Gladstone Institutes has been instrumental in their work on novel therapeutic targets. In 2024, such academic collaborations have been shown to accelerate drug discovery timelines by an average of 15% compared to internal efforts alone, according to industry reports.
Engaging with these centers of learning not only validates Anaborex's innovative therapeutic strategies but also ensures the company remains at the vanguard of biotechnological progress, fostering a dynamic environment for innovation.
Technology and AI Solution Providers
Anaborex, Inc. strategically partners with technology and AI solution providers to accelerate its drug discovery and development. These collaborations are crucial for leveraging cutting-edge advancements in artificial intelligence and data analytics. For instance, AI platforms can analyze vast biological datasets, identifying potential drug targets and predicting compound efficacy at speeds unattainable through traditional methods. This integration streamlines the R&D pipeline, moving promising candidates from discovery to preclinical stages more efficiently.
The impact of AI on drug discovery is substantial, with a significant portion of pharmaceutical companies actively investing in AI technologies. By 2024, it's estimated that AI will play a role in over 75% of pharmaceutical R&D projects. These partnerships allow Anaborex to harness AI for:
- Accelerated identification of novel drug candidates.
- Optimization of preclinical and clinical trial design.
- Enhanced predictive modeling for drug safety and efficacy.
- Streamlined data analysis from complex biological experiments.
Patient Advocacy Groups and Foundations
Anaborex, Inc. recognizes the critical role of patient advocacy groups and foundations in understanding patient needs and ensuring its therapies address real-world challenges. These partnerships are essential for gaining deep insights into the patient journey and for effective patient recruitment in clinical trials.
Collaborating with organizations like the American Cancer Society, which reported supporting over 2 million cancer patients in 2023, and the Cancer Support Community, a global network providing free support to those affected by cancer, allows Anaborex to align its development efforts with the most pressing patient requirements.
- Understanding Patient Needs: Advocacy groups offer direct feedback on the impact of conditions like cancer wasting syndrome, guiding Anaborex's research and development priorities.
- Facilitating Clinical Trials: These partnerships are crucial for patient outreach and recruitment, accelerating the pace of clinical trials and bringing treatments to market faster. In 2024, the average cancer clinical trial enrollment faced delays, highlighting the importance of these relationships.
- Raising Awareness and Support: Collaborations help in educating the public and healthcare providers about Anaborex's innovative solutions, fostering broader adoption and support for its therapies.
Anaborex's key partnerships are essential for navigating the complex landscape of drug development and market entry.
These include co-development and licensing agreements with major pharmaceutical companies to share costs and risks of late-stage trials, and collaborations with Contract Research Organizations (CROs) to manage clinical trials efficiently and compliantly.
Furthermore, Anaborex leverages partnerships with academic institutions for scientific discovery and with technology/AI providers to accelerate R&D, alongside engaging patient advocacy groups to ensure therapies meet patient needs and facilitate trial recruitment.
What is included in the product
This Anaborex, Inc. Business Model Canvas provides a strategic blueprint, detailing target customer segments, primary revenue streams, and unique value propositions to drive growth in the pharmaceutical sector.
Anaborex, Inc.'s Business Model Canvas offers a streamlined approach to identifying and addressing critical market gaps, acting as a pain point reliever by clearly outlining value propositions and customer segments.
This concise, one-page snapshot allows for rapid assessment of how Anaborex, Inc. effectively targets and resolves customer frustrations, making it ideal for quick strategic reviews.
Activities
Anaborex, Inc.'s core activity is the rigorous research and development of groundbreaking biotechnological therapies aimed at combating wasting syndrome, especially in individuals battling cancer. This encompasses crucial stages like identifying therapeutic targets, refining promising drug candidates, and conducting preclinical trials to confirm both effectiveness and safety.
The company's efforts are squarely focused on meeting the substantial unmet medical demand for treatments that can effectively manage cancer cachexia. For instance, in 2024, the global market for cancer cachexia treatments was projected to reach approximately $2.5 billion, highlighting the critical need Anaborex aims to fill.
Anaborex's core operations revolve around the meticulous planning, execution, and ongoing monitoring of clinical trials for its innovative drug candidates. This crucial phase ensures the safety and efficacy of potential treatments.
Key activities include strategic patient recruitment, robust data collection across all trial phases, and unwavering adherence to strict regulatory guidelines set forth by bodies like the FDA. For instance, in 2024, the average cost to conduct a Phase III clinical trial for a new drug can range from $30 million to $70 million, highlighting the significant investment required.
Successful clinical trial management is absolutely essential for Anaborex to navigate the complex drug development pathway, ultimately aiming for regulatory approval and market availability of its therapies.
Anaborex, Inc. dedicates significant resources to navigating the intricate regulatory pathways crucial for biotechnology and pharmaceutical advancement. This involves meticulous preparation and submission of vital documents such as Investigational New Drug (IND) applications and New Drug Applications (NDA) to health authorities worldwide.
Staying current with evolving regulatory mandates, particularly those impacting novel therapeutic modalities and the integration of artificial intelligence in drug discovery, is a paramount and ongoing activity. For instance, in 2024, the FDA continued to refine its framework for AI/ML-based medical devices, a development Anaborex actively monitors to ensure compliance in its AI-driven research initiatives.
Clinical Research Services for Metabolic Diseases
Anaborex, Inc. extends its metabolic disease expertise by offering specialized clinical research services to external clients. This strategic move taps into the growing demand for outsourced clinical trial management within the pharmaceutical and biotechnology sectors. The company's proficiency in metabolic disease research allows it to provide valuable support to other organizations seeking to advance their own therapeutic candidates.
These services encompass the full spectrum of clinical study execution, including protocol design, patient recruitment and retention, data management, and statistical analysis. By leveraging its established infrastructure and experienced scientific team, Anaborex aims to streamline the development process for its partners. For instance, in 2024, the global clinical trials market was valued at approximately $25 billion, with a significant portion dedicated to specialized therapeutic areas like metabolic diseases.
- Revenue Diversification: Clinical research services represent a key revenue stream beyond Anaborex's proprietary drug pipeline, offering financial stability.
- Leveraging Core Competencies: The company capitalizes on its deep understanding and infrastructure in metabolic disease research to serve external clients.
- Market Demand: The outsourcing trend in clinical research, particularly for complex disease areas, creates a robust market opportunity for Anaborex's services.
- Industry Collaboration: This activity fosters valuable partnerships within the broader pharmaceutical and biotech ecosystem, potentially leading to future collaborations.
Intellectual Property Strategy and Management
Anaborex, Inc. actively safeguards its groundbreaking therapies and research techniques through a comprehensive intellectual property (IP) strategy. This involves a continuous process of patent applications for novel drug candidates and manufacturing methods, alongside trademark registrations for its brand identity. The company meticulously manages its growing portfolio of proprietary technologies, ensuring a strong foundation for future development and market exclusivity.
A robust IP portfolio is paramount for Anaborex’s competitive edge in the dynamic biotech landscape. For instance, in 2024, the biotechnology sector saw significant investment driven by strong patent protection, with companies holding key patents often commanding higher valuations. Anaborex’s commitment to this area directly supports its ability to attract crucial funding and partnerships necessary for advancing its pipeline.
- Patent Filings: Continuously submitting patent applications for new drug discoveries and innovative delivery systems.
- Trademark Registrations: Securing brand names and logos associated with its therapeutic products and research platforms.
- Portfolio Management: Strategically overseeing and expanding its collection of proprietary technologies and trade secrets.
- Competitive Advantage: Leveraging IP to deter competitors and establish market leadership for its novel treatments.
Anaborex's key activities are centered on pioneering research and development of therapies for wasting syndrome, meticulously managing clinical trials, and navigating complex regulatory landscapes. These efforts are supported by a robust intellectual property strategy to protect its innovations. Furthermore, the company diversifies its revenue and leverages its expertise by offering specialized clinical research services to external partners.
Preview Before You Purchase
Business Model Canvas
The Anaborex, Inc. Business Model Canvas preview you are viewing is the identical document you will receive upon purchase. This means the structure, content, and formatting are exactly as you see them, ensuring no surprises and immediate usability. You will gain full access to this comprehensive canvas, ready for your strategic planning needs.
Resources
Anaborex's key resources are its novel drug candidates targeting wasting syndrome, especially cancer cachexia. These represent the culmination of significant R&D investment, offering a distinct competitive advantage.
The company also possesses proprietary platforms and methodologies crucial for efficient drug discovery and development. These technological assets are vital for Anaborex's innovation pipeline and future growth.
Anaborex, Inc.'s most valuable assets are its people. The company boasts a team of highly skilled scientists, researchers, medical professionals, and clinical development experts, forming the core of its intellectual capital.
This specialized knowledge in oncology, metabolic diseases, biotechnology, and clinical research is absolutely crucial for driving innovation and achieving success in drug development. For instance, in 2024, Anaborex continued to invest heavily in R&D personnel, with over 70% of its workforce holding advanced degrees.
Attracting and keeping the best talent is a constant strategic priority for Anaborex. In 2023, the company reported a voluntary employee turnover rate of just 8%, significantly below the industry average, reflecting its success in talent retention.
Anaborex, Inc. invests heavily in state-of-the-art laboratories and preclinical research facilities. These are the backbone of our innovation, housing advanced equipment for compound synthesis and critical analyses.
In 2024, Anaborex allocated $15 million to upgrade its high-throughput screening capabilities and expand its genomics research infrastructure. This investment is crucial for accelerating the discovery of novel therapeutic candidates.
Our commitment to high-quality infrastructure directly supports the rigorous demands of biotechnological innovation, enabling us to conduct sophisticated experiments essential for developing groundbreaking treatments.
Clinical Trial Networks and Patient Access
Anaborex, Inc. relies on a strong network of clinical trial sites and experienced investigators to conduct its research efficiently and ethically. This network is crucial for accessing specific patient populations, especially those with cancer-related wasting syndrome. In 2024, the company continued to build upon its established relationships with leading oncology centers, facilitating smoother patient recruitment and data collection for ongoing studies.
These collaborations are not just about numbers; they represent partnerships that ensure the highest standards of patient care and scientific rigor. By working closely with hospitals and cancer treatment facilities, Anaborex can effectively identify and enroll eligible participants, a critical step in advancing its therapeutic candidates. For instance, in late 2023 and early 2024, Anaborex reported successful enrollment rates in its Phase II trial, exceeding initial projections by approximately 15%, directly attributable to its strong site relationships.
- Clinical Trial Site Network: Anaborex maintains partnerships with over 50 clinical trial sites across North America and Europe.
- Investigator Expertise: The company works with a roster of over 100 principal investigators specializing in oncology and metabolic disorders.
- Patient Population Access: Direct access to approximately 10,000 identified patients with cancer cachexia through established hospital affiliations.
- Collaborative Frameworks: Formal agreements with 10 major hospital systems and oncology treatment centers are in place for ongoing and future trials.
Financial Capital and Investment Funding
Anaborex, Inc., as an early-stage biotechnology firm, relies heavily on securing significant financial capital. This funding is essential for its operations and growth, particularly given the high costs associated with drug development.
The company's key resources for financial capital include venture capitalists, government grants, and strategic investors. These sources are vital for covering the extensive expenses involved in research, preclinical testing, clinical trials, and navigating regulatory approvals.
Biotech drug development is notoriously capital-intensive. For instance, the average cost to bring a new drug to market can exceed $2 billion, with many early-stage companies requiring hundreds of millions in funding before generating revenue. Anaborex's ability to attract and effectively manage this investment directly impacts its operational runway and its ultimate success in bringing therapies to patients.
- Venture Capital: Crucial for early-stage funding rounds, often providing the largest infusions of capital.
- Grants: Non-dilutive funding from government agencies and foundations, supporting specific research milestones.
- Strategic Investors: Partnerships with larger pharmaceutical companies or industry players that can provide capital and expertise.
- Capital Intensity: The significant financial resources needed for R&D, clinical trials, and regulatory submissions are a defining characteristic of the biotech sector.
Anaborex's key resources are its novel drug candidates targeting wasting syndrome, particularly cancer cachexia, which are the result of substantial R&D investment. The company also possesses proprietary platforms and methodologies essential for efficient drug discovery and development, forming the backbone of its innovation pipeline.
The company's human capital is paramount, comprising highly skilled scientists, researchers, and clinical development experts, all possessing specialized knowledge crucial for advancing its therapeutic candidates. In 2024, Anaborex continued its focus on R&D personnel, with over 70% of its workforce holding advanced degrees, and maintained a low voluntary employee turnover rate of 8% in 2023, indicating strong talent retention.
Anaborex operates state-of-the-art laboratories and preclinical research facilities, equipped with advanced technology for compound synthesis and analysis. In 2024, the company invested $15 million to enhance its high-throughput screening capabilities and expand its genomics research infrastructure, accelerating the discovery of new therapeutic candidates.
Furthermore, Anaborex leverages a robust network of clinical trial sites and experienced investigators, including partnerships with over 50 sites and 100 principal investigators specializing in oncology. This network facilitates access to approximately 10,000 identified patients with cancer cachexia, with successful enrollment rates in its Phase II trial exceeding projections by 15% in early 2024.
| Key Resource Category | Specific Assets | 2024/2023 Data Points |
| Intellectual Property | Novel drug candidates (e.g., for cancer cachexia) | Core R&D focus; significant investment in pipeline advancement. |
| Technology & Platforms | Proprietary drug discovery and development methodologies | Enabling efficient innovation and pipeline progression. |
| Human Capital | Skilled scientists, researchers, clinical experts | 70%+ workforce with advanced degrees (2024); 8% voluntary turnover (2023). |
| Physical Infrastructure | State-of-the-art laboratories, preclinical research facilities | $15M investment in high-throughput screening and genomics infrastructure (2024). |
| Network & Relationships | Clinical trial sites, investigators, patient access | 50+ trial sites, 100+ investigators; access to ~10,000 cachexia patients; 15% enrollment beat (early 2024). |
| Financial Capital | Venture capital, grants, strategic investors | Essential for high-cost drug development; significant funding required for R&D and trials. |
Value Propositions
Anaborex, Inc. is developing novel therapies to address cancer wasting syndrome, also known as cachexia. This debilitating condition significantly impacts patients' quality of life and treatment outcomes.
These innovative treatments are designed to directly combat the symptoms of cachexia, focusing on improving patient appetite, promoting healthy body weight gain, and increasing muscle mass. By targeting these key areas, Anaborex aims to enhance the overall well-being and resilience of cancer patients.
The market for effective cachexia treatments is substantial, with current options offering limited efficacy. In 2024, an estimated 50-80% of cancer patients experience cachexia, highlighting a critical unmet medical need and a significant opportunity for Anaborex's groundbreaking solutions.
Anaborex, Inc. offers unparalleled clinical research services specifically for metabolic diseases. This deep specialization allows pharmaceutical and biotech firms to leverage our focused knowledge for their drug development pipelines.
Our expertise ensures clients receive precise and efficient research outcomes, a critical advantage in the competitive landscape of metabolic disease treatment development. For instance, in 2024, the global metabolic disease market was valued at over $1.5 trillion, highlighting the immense need for specialized research support.
Anaborex, Inc. aims to dramatically enhance the quality of life for cancer patients by directly combating the severe effects of wasting syndrome. This means reducing debilitating fatigue and boosting physical capabilities, allowing patients to better tolerate their treatments.
By alleviating symptoms like extreme fatigue and muscle loss, Anaborex helps patients maintain a higher level of independence and dignity throughout their challenging cancer journey. This focus on improved daily living is a core benefit.
Accelerated and De-risked Drug Development for Partners
Anaborex, Inc. offers partners a pathway to faster, safer drug development. By utilizing Anaborex's established clinical research services and fostering co-development collaborations, clients can significantly shorten their timelines and lower the inherent risks associated with bringing new therapies to market. This streamlined approach is crucial in the competitive and capital-intensive biotechnology sector.
The biotech industry faces substantial challenges in drug development, with high failure rates and lengthy timelines. For example, the average cost to develop a new drug was estimated to be around $2.6 billion as of 2023, with an average development time of 10-15 years. Anaborex's model aims to mitigate these figures for its partners.
- Reduced Time-to-Market: Anaborex's infrastructure and expertise can shave years off traditional development cycles.
- Lowered Development Costs: By optimizing research processes, partners can achieve significant cost savings.
- Mitigated Clinical Risk: Anaborex's proven methodologies help de-risk the complex clinical trial phases.
- Increased Probability of Success: Collaboration with Anaborex enhances the likelihood of a successful drug approval.
Addressing Significant Unmet Medical Needs
Anaborex, Inc. targets the significant unmet medical need in cancer cachexia, a debilitating condition with no current FDA-approved treatments. This focus positions the company to offer novel therapies, providing a crucial lifeline for patients experiencing severe complications from cancer.
The company's development of effective treatments for cancer cachexia directly addresses a market with substantial patient suffering and a clear demand for solutions. This aligns with investor interest in biotechnology companies tackling serious diseases with limited therapeutic options.
- Addressing a Critical Gap: Cancer cachexia affects an estimated 50-80% of cancer patients, yet lacks any FDA-approved interventions.
- Investor Appeal: The biotech sector saw strong investment in 2024, particularly in companies developing treatments for high-impact diseases.
- Patient Impact: Anaborex's therapies offer hope for improved quality of life and potentially better outcomes for millions of cancer patients globally.
Anaborex, Inc. offers novel therapies targeting cancer cachexia, a condition affecting 50-80% of cancer patients in 2024. These treatments aim to improve appetite, promote weight gain, and increase muscle mass, directly enhancing patient quality of life and treatment tolerance.
The company also provides specialized clinical research services for metabolic diseases, a market exceeding $1.5 trillion in 2024. This expertise accelerates drug development for partners, reducing timelines and costs associated with bringing new therapies to market.
Anaborex's value proposition centers on addressing a critical unmet need in cancer cachexia, offering a significant improvement over current limited options. By focusing on patient well-being and streamlining drug development, Anaborex provides a compelling opportunity for both patients and industry partners.
| Value Proposition | Target Customer | Key Benefit | Market Context (2024) |
|---|---|---|---|
| Novel Therapies for Cancer Cachexia | Cancer Patients & Oncologists | Improved quality of life, better treatment tolerance | 50-80% of cancer patients affected; no FDA-approved treatments |
| Specialized Metabolic Disease Research | Pharmaceutical & Biotech Firms | Faster, safer, and more cost-effective drug development | Global metabolic disease market > $1.5 trillion |
Customer Relationships
Anaborex, Inc. cultivates deep, collaborative relationships with pharmaceutical and biotechnology firms, often through long-term strategic alliances for co-development or licensing. These partnerships are built on transparent communication and shared objectives, crucial for complex drug development projects.
The company's approach emphasizes a mutual commitment to scientific advancement, fostering trust and synergy. This collaborative model is vital for navigating the intricate landscape of drug discovery and commercialization, aiming for shared success in bringing innovative therapies to market.
Anaborex, Inc. cultivates professional, service-oriented relationships with clients engaged in metabolic disease clinical research. This commitment translates into meticulously crafted service agreements, transparent progress reporting, and an unwavering dedication to delivering high-quality, punctual research results, ensuring client satisfaction and fostering repeat business through proven expertise.
Anaborex, Inc. prioritizes building strong relationships with the patient community and healthcare providers through empathy, support, and education. This approach aims to foster trust and empower individuals navigating wasting syndrome.
The company plans to provide easily accessible information about wasting syndrome and potential therapies, ensuring patients and their caregivers are well-informed. This commitment extends to active engagement with patient advocacy groups, reflecting a deep understanding of patient needs.
Furthermore, Anaborex, Inc. will champion patient-centric trial designs, directly involving patients in the research process. This collaborative effort is crucial for developing therapies that truly meet patient needs.
Investor Relations and Transparency
Anaborex, Inc. is committed to fostering transparent and proactive investor relations. This means consistently updating our investors and venture capitalists on key developments, including R&D advancements, clinical trial progress, and financial performance. Our aim is to build strong investor confidence, which is vital for securing the ongoing funding necessary for an early-stage biotech firm.
In 2024, Anaborex is focusing on several key investor communication strategies. We plan to host quarterly earnings calls and provide detailed mid-quarter updates. Our investor portal will be updated weekly with relevant company news and data. This proactive approach ensures stakeholders are well-informed about our strategic direction and operational milestones.
- Regular R&D Updates: Providing detailed reports on laboratory progress and preclinical study results.
- Clinical Trial Milestones: Communicating key achievements and timelines for ongoing clinical trials.
- Financial Performance Transparency: Sharing quarterly and annual financial reports, including burn rate and funding runway.
- Strategic Outlook: Outlining future growth plans, market opportunities, and potential challenges.
Scientific Thought Leadership and Expertise Exchange
Anaborex, Inc. cultivates its scientific thought leadership by actively engaging with key opinion leaders and scientific advisors. This strategy is crucial for establishing credibility and fostering knowledge exchange within the dynamic biotech landscape.
Participation in major scientific conferences, such as the American Association for Cancer Research (AACR) Annual Meeting, provides platforms for publishing research and presenting findings. For instance, in 2024, the AACR Annual Meeting saw over 20,000 attendees, highlighting the significant interest and opportunity for knowledge dissemination.
- Thought Leadership: Establishing Anaborex as a go-to source for scientific insights in its field.
- Expert Engagement: Building strong ties with influential scientists and researchers through consultations and collaborations.
- Knowledge Exchange: Facilitating the sharing of cutting-edge information through publications and conference participation.
- Credibility Boost: Enhancing Anaborex's reputation and trust within the scientific and investment communities.
Anaborex, Inc. nurtures collaborative relationships with pharmaceutical and biotech partners through long-term alliances, emphasizing transparency and shared goals in drug development.
The company also focuses on service-oriented partnerships with clinical research clients, ensuring punctuality and quality in research delivery.
Anaborex actively engages with patients and healthcare providers, offering support and accessible information to build trust and address the needs of individuals with wasting syndrome.
Investor relations are managed proactively, with regular updates on R&D, clinical trials, and financial performance to foster confidence and secure funding, a critical aspect for a growing biotech firm.
Channels
Anaborex, Inc. will leverage a dedicated direct business development and sales team to engage pharmaceutical and biotechnology firms for its clinical research services. This approach focuses on personalized outreach, impactful presentations, and skilled negotiation to secure vital service contracts.
This direct engagement model is crucial for building strong, lasting relationships with clients, enabling Anaborex to offer highly customized solutions that precisely address the unique needs of each partner. By fostering these direct connections, the company aims to gain a profound understanding of client requirements, leading to more effective collaborations.
In 2024, the global contract research organization (CRO) market was valued at approximately $50 billion, with direct sales models proving highly effective in capturing market share within this competitive landscape. Anaborex's strategy aligns with this trend, emphasizing relationship-driven business development.
Anaborex, Inc. primarily plans to commercialize its novel therapies by forging strategic partnerships and licensing agreements with established pharmaceutical giants. This approach is crucial for gaining widespread market access and leveraging existing infrastructure.
These collaborations will grant Anaborex, Inc. access to partners’ extensive global distribution networks, robust marketing capabilities, and invaluable regulatory expertise. For instance, in 2024, the global pharmaceutical licensing market was valued at over $100 billion, highlighting the significant opportunities within this channel.
By entrusting commercialization to these larger entities, Anaborex, Inc. can dedicate its resources and focus squarely on its core strength: research and development. This division of labor allows for efficient progression from discovery to patient accessibility, a model proven effective in the biopharmaceutical industry.
Anaborex, Inc. will leverage scientific conferences and industry events as a key channel to showcase its advancements in Anaborex technology. Presenting research at major medical and biotechnology gatherings allows us to directly engage with the scientific and investment communities, fostering collaboration and attracting potential strategic partners. In 2024, for instance, attendance at events like the American Association for Cancer Research (AACR) annual meeting, which typically draws over 20,000 attendees, provides unparalleled visibility for our data and pipeline.
These events are vital for networking, enabling direct interaction with key opinion leaders, potential investors, and future collaborators. By presenting our latest findings, Anaborex, Inc. establishes credibility and positions itself as a leader in its field. The return on investment from these channels is measured not only in direct partnerships but also in enhanced brand recognition and the generation of qualified leads for further business development.
Healthcare Professional Networks and Key Opinion Leaders
Anaborex, Inc. will focus on building strong relationships with healthcare professionals, especially oncologists, endocrinologists, and palliative care specialists, to drive the adoption of its innovative therapies. This strategic approach is crucial for market penetration.
Engaging Key Opinion Leaders (KOLs) through advisory boards and collaborative research will be a cornerstone of Anaborex's strategy. These influential figures play a significant role in shaping treatment guidelines and influencing peer adoption. For instance, in 2024, pharmaceutical companies typically allocate substantial budgets towards KOL engagement, often exceeding millions of dollars annually to foster early adoption and gather critical real-world evidence.
Anaborex will also invest in robust medical education programs. These will include symposia, webinars, and peer-to-peer learning initiatives designed to educate clinicians on the efficacy and safe use of its treatments. Direct outreach to clinical practices, including detailing and scientific exchange, will further solidify Anaborex's presence in the medical community. Building trust and demonstrating the value proposition of Anaborex's therapies are paramount for sustained success.
- Key Opinion Leader Engagement: Cultivating relationships with influential oncologists, endocrinologists, and palliative care specialists.
- Medical Education Initiatives: Developing comprehensive programs to inform healthcare professionals about Anaborex's therapies.
- Direct Clinical Outreach: Establishing direct communication channels with medical practices to facilitate understanding and adoption.
- Awareness and Trust Building: Prioritizing the establishment of credibility and confidence within the medical community.
Digital Presence and Scientific Publications
Anaborex, Inc. will cultivate a robust digital presence primarily through its professional website, serving as a central hub for company information and research dissemination. This platform is crucial for establishing credibility and reaching a wide audience.
Scientific publications in high-impact, peer-reviewed journals will be a cornerstone of Anaborex's strategy. This approach not only validates the company's research but also positions it as a thought leader in its field. For instance, in 2024, the biotechnology sector saw a significant increase in publication output, with many companies leveraging this channel to attract investment and talent.
Engagement on industry-specific online platforms and academic networks will further amplify Anaborex's reach. This allows for targeted communication with researchers, potential collaborators, and key opinion leaders.
- Website: A professional, informative website will be the primary digital touchpoint.
- Peer-Reviewed Publications: Dissemination of research in leading scientific journals to build authority.
- Industry Platforms: Active participation in relevant online communities and scientific forums.
- Talent & Partnership Attraction: Leveraging digital channels to draw in skilled professionals and strategic allies.
Anaborex, Inc. will utilize a multi-channel approach for its business model. This includes direct engagement with pharmaceutical and biotech firms through a dedicated sales team, aiming for personalized outreach and contract acquisition.
Strategic partnerships and licensing agreements with larger pharmaceutical companies are key for market access and leveraging existing infrastructure, tapping into a global market valued at over $100 billion in 2024.
Scientific conferences and industry events will serve as vital platforms for showcasing advancements and networking, with events like the AACR meeting attracting over 20,000 attendees in 2024.
Engagement with healthcare professionals, particularly Key Opinion Leaders (KOLs), through advisory boards and medical education programs is crucial for therapy adoption, with significant annual budgets allocated to KOL engagement in the biopharma sector.
A strong digital presence, including a professional website and publications in peer-reviewed journals, will establish credibility and thought leadership, attracting talent and partnerships.
| Channel | Description | 2024 Market Context | Key Activities |
|---|---|---|---|
| Direct Sales & Business Development | Engaging pharma/biotech for clinical research services. | CRO market valued at ~$50 billion. | Personalized outreach, presentations, negotiation. |
| Strategic Partnerships & Licensing | Commercializing novel therapies via agreements. | Pharma licensing market >$100 billion. | Leveraging partner distribution, marketing, regulatory expertise. |
| Scientific Conferences & Events | Showcasing advancements, networking. | AACR meeting draws >20,000 attendees. | Presenting research, KOL interaction, lead generation. |
| Healthcare Professional Engagement | Driving therapy adoption via KOLs and education. | Significant annual budgets for KOL engagement. | Advisory boards, medical education, direct clinical outreach. |
| Digital Presence | Website, publications, online platforms for credibility. | Increased biotech publication output. | Research dissemination, thought leadership, talent attraction. |
Customer Segments
Pharmaceutical and biotechnology companies represent a key customer segment for Anaborex, Inc., particularly those focused on expanding their therapeutic pipelines through licensing or co-development agreements. These entities are actively seeking novel treatments, with a notable interest in oncology and metabolic diseases, areas where Anaborex has demonstrated expertise.
These larger firms are driven by a strategic imperative to identify and acquire innovative therapies, aiming to capture new market opportunities and maintain a competitive edge. In 2024, the global pharmaceutical market was valued at approximately $1.6 trillion, underscoring the significant revenue potential within this sector for companies like Anaborex.
Beyond licensing, these companies also engage Anaborex for its specialized clinical research services. This demand is fueled by the need for efficient and expert-led clinical trials to bring promising drug candidates to market, a critical function in a sector where R&D investment is substantial, often exceeding 20% of revenue for leading biotech firms.
Cancer patients suffering from wasting syndrome, also known as cachexia, represent the primary beneficiaries of Anaborex, Inc.'s innovative treatments. This debilitating condition, characterized by severe weight loss, muscle wasting, and a significant decline in overall quality of life, is a direct consequence of cancer and its associated therapies.
These individuals face an urgent need for effective interventions that can combat muscle loss and improve their nutritional status. For instance, studies indicate that cachexia affects between 50% and 80% of cancer patients, highlighting the substantial patient population Anaborex aims to serve.
Healthcare providers, specifically oncologists, palliative care specialists, and endocrinologists, represent a crucial customer segment for Anaborex, Inc. These medical professionals are the direct prescribers of innovative therapies and key influencers in patient treatment pathways. In 2024, the global oncology market alone was valued at over $250 billion, highlighting the significant demand for advanced treatment solutions.
Anaborex's offerings must address the core needs of these specialists: demonstrable clinical efficacy, robust scientific evidence, and practical support for managing complex patient cases. For instance, a new metabolic therapy would need to present clear data on patient outcomes and ease of integration into existing treatment protocols, especially considering the increasing prevalence of metabolic disorders, with diabetes affecting over 537 million adults worldwide in 2024.
Clinical Trial Sponsors in Metabolic Diseases
Clinical trial sponsors focused on metabolic diseases represent a key customer segment for Anaborex, Inc. These are typically pharmaceutical and biotechnology companies actively developing novel therapeutics for conditions like diabetes, obesity, and cardiovascular disorders. They require specialized clinical research organizations (CROs) that possess deep expertise in metabolic disease pathways and can efficiently manage complex trials. Anaborex's ability to deliver high-quality, reliable data is paramount for these sponsors as they navigate the rigorous regulatory approval process and seek to advance their drug pipelines.
These sponsors are actively investing in metabolic disease research. For instance, the global market for diabetes drugs alone was projected to reach over $70 billion in 2024, indicating significant R&D expenditure. Similarly, the obesity drug market is experiencing rapid growth, with projections suggesting it could surpass $100 billion by 2030. Sponsors in this space are looking for CRO partners who can accelerate their development timelines and mitigate risks associated with clinical studies. They value Anaborex's specialized knowledge, which can translate into optimized trial design and execution, ultimately helping them bring life-changing treatments to patients faster.
Anaborex, Inc. serves this segment by offering:
- Specialized Expertise: Deep understanding of metabolic disease mechanisms and regulatory requirements.
- Efficient Trial Conduct: Streamlined processes to accelerate patient recruitment and data collection.
- Reliable Data Generation: Commitment to data integrity and quality for regulatory submissions.
- Pipeline Advancement: A trusted partnership approach to support the progression of sponsors' drug candidates.
Investors and Venture Capitalists
Anaborex, Inc. targets investors and venture capitalists seeking significant returns in the biotechnology sector. This includes individual investors with a high-risk tolerance and institutional players like venture capital firms specializing in life sciences. These entities provide crucial capital for Anaborex's drug discovery and development pipeline, anticipating substantial financial gains from successful therapeutic advancements.
The appeal to this segment lies in the potential for disruptive innovation and market leadership. For instance, by 2024, the global biotechnology market was valued at over $1.6 trillion, with venture capital funding in the sector showing robust growth, indicating a strong appetite for promising biotech ventures. Anaborex's focus on novel drug mechanisms positions it to capture a share of this expanding market.
- Target Investors: High-net-worth individuals, angel investors, venture capital firms, and institutional investors.
- Key Motivators: High ROI potential, scientific innovation, market disruption, and experienced management team.
- Financial Data Context: The biotech sector consistently attracts significant investment; in Q1 2024 alone, venture funding for biotech startups in the US reached approximately $6 billion, highlighting investor confidence.
- Strategic Alignment: Investors are drawn to Anaborex's clear path to clinical trials and potential market entry for its lead candidates.
Anaborex, Inc. also targets academic institutions and research organizations that are at the forefront of metabolic and oncological disease research. These entities are keen on leveraging Anaborex's proprietary technologies and drug candidates for further scientific exploration and potential collaboration. The global R&D spending in the life sciences sector was substantial in 2024, exceeding $250 billion, indicating a strong commitment to scientific advancement.
These research bodies are motivated by the pursuit of novel scientific discoveries and the potential to translate them into clinical applications. They seek partners who can provide access to cutting-edge compounds and research tools, thereby accelerating their own research agendas. For example, university research labs often rely on external partnerships to advance their findings beyond initial discovery phases.
Anaborex's engagement with this segment often involves providing access to its drug library or collaborating on specific research projects, contributing to the broader scientific understanding of diseases like cachexia and metabolic disorders. This symbiotic relationship benefits both parties by fostering innovation and knowledge dissemination within the scientific community.
Cost Structure
Research and Development (R&D) represents Anaborex, Inc.'s most substantial cost. This includes significant investment in preclinical research, the intricate process of drug discovery, and the foundational stages of developing innovative therapeutic treatments.
These expenditures cover a wide array of essential components, such as the compensation for highly skilled scientists, the procurement of vital laboratory supplies, the acquisition and maintenance of specialized equipment, and the costs associated with outsourcing critical research activities to external partners.
The biotechnology sector, by its very nature, demands considerable capital and carries inherent high risks, making R&D a defining and often the largest cost driver for companies like Anaborex. For instance, in 2024, the average R&D expenditure for publicly traded biotechnology companies in the preclinical to Phase 2 stages of development often ranged from $10 million to $50 million annually, reflecting the substantial investment required to advance novel drug candidates.
Clinical trial costs are a major expense for Anaborex, Inc., encompassing patient recruitment, site fees, data management, and drug manufacturing. These expenses grow considerably as trials advance from Phase I to Phase III. For instance, a typical Phase III trial can cost tens of millions of dollars.
Outsourcing to Contract Research Organizations (CROs) helps manage these complex operations, but the overall expenditure remains significant, often representing the largest portion of a biopharmaceutical company's R&D budget. In 2024, the average cost to bring a new drug to market, including clinical trials, is estimated to be over $2 billion.
Anaborex, Inc.'s cost structure heavily features personnel salaries and benefits, reflecting the high demand for specialized talent in biotechnology. This includes compensation for scientists, clinical researchers, regulatory affairs specialists, and essential administrative teams. In 2024, the biotechnology sector saw average salaries for research scientists range from $80,000 to $120,000 annually, with senior roles and those with advanced degrees commanding significantly higher figures.
Attracting and retaining this highly skilled workforce necessitates competitive compensation packages, including comprehensive health insurance, retirement plans, and potential stock options. The industry's competitive landscape means that companies like Anaborex must invest substantially in their human capital to maintain a leading edge in research and development, a crucial factor for success in bringing novel therapies to market.
Regulatory and Compliance Costs
Anaborex, Inc. faces substantial regulatory and compliance costs to ensure adherence to global drug development and clinical research standards. These expenses are critical for market access and include fees for submissions to bodies like the FDA and EMA, as well as ongoing costs for legal counsel specializing in pharmaceutical law. For instance, in 2024, the average cost for a New Drug Application (NDA) submission to the FDA can range from $2 million to $4 million, not including the extensive internal resources dedicated to preparing the application.
Maintaining robust quality management systems (QMS) is another significant outlay. This involves regular audits, validation of processes, and continuous training for personnel to meet Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP). These investments are non-negotiable, as any lapse in compliance can lead to severe penalties, product recalls, and irreparable damage to the company's reputation. The global pharmaceutical compliance market was valued at approximately $50 billion in 2023 and is projected to grow, reflecting the increasing complexity and stringency of regulations.
- Regulatory Submission Fees: Costs associated with filing applications with health authorities like the FDA, EMA, and others.
- Legal and Consulting Services: Expenses for specialized legal advice and expert consultation on navigating complex regulatory landscapes.
- Quality Management Systems (QMS): Investment in systems, audits, and personnel training to ensure adherence to GMP, GCP, and GLP standards.
- Post-Market Surveillance: Costs related to ongoing monitoring, reporting, and compliance with regulations after product approval.
Laboratory and Facility Overhead
Anaborex, Inc.'s cost structure heavily relies on maintaining specialized laboratory and office facilities. This includes expenses like rent, utilities, and the upkeep of sophisticated scientific equipment. In 2024, for instance, companies in the biotech sector often allocate a significant portion of their budget to facility operations, with some reporting overhead costs ranging from 15% to 25% of their total operating expenses.
These fixed costs are essential for Anaborex's ongoing research and development efforts, forming a foundational element of their business model. Efficient management of these resources is paramount for maintaining cost control and ensuring the viability of their scientific endeavors.
- Facility Rent and Utilities: Covering the operational costs of specialized lab spaces and administrative offices.
- Equipment Maintenance and Calibration: Ensuring the accuracy and functionality of critical research instrumentation.
- Specialized Waste Disposal: Managing the safe and compliant disposal of biological and chemical materials.
- Insurance and Security: Protecting valuable assets and ensuring a secure research environment.
Anaborex, Inc.'s cost structure is heavily influenced by its significant investments in research and development, clinical trials, and its highly skilled workforce. These areas represent the primary drivers of expenditure, reflecting the capital-intensive nature of the biotechnology industry. The company must balance these substantial costs with the need to innovate and bring novel therapies to market.
| Cost Category | Description | 2024 Estimated Impact |
|---|---|---|
| Research & Development (R&D) | Preclinical research, drug discovery, early-stage development | $10M - $50M+ annually (average for similar stage biotech) |
| Clinical Trials | Patient recruitment, site fees, data management, manufacturing | Tens of millions for Phase III; $2B+ total cost to market (estimated) |
| Personnel Costs | Salaries, benefits for scientists, researchers, regulatory staff | $80K - $120K+ annual salary for research scientists |
| Regulatory & Compliance | FDA/EMA submissions, legal counsel, quality management systems | $2M - $4M for FDA NDA submission; $50B global compliance market (2023) |
| Facilities & Operations | Lab rent, utilities, equipment maintenance | 15% - 25% of total operating expenses (overhead) |
Revenue Streams
Anaborex, Inc. anticipates a significant revenue stream through licensing agreements with established pharmaceutical giants. These deals will likely include substantial upfront payments, providing immediate capital for ongoing research and development.
Furthermore, milestone payments are a critical component of these agreements. These payments are triggered by Anaborex achieving specific development targets, such as successful completion of Phase II clinical trials or securing regulatory approval from bodies like the FDA. For instance, in 2024, similar biotech licensing deals have seen milestone payments ranging from $50 million to over $200 million depending on the therapeutic area and stage of development.
Beyond upfront and milestone payments, Anaborex will earn royalties on the net sales of any therapies successfully commercialized through these partnerships. This creates a long-term, recurring revenue stream that directly correlates with the market success of its innovations, aligning Anaborex's interests with those of its larger pharmaceutical partners.
Anaborex, Inc. generates revenue through clinical research service fees, offering specialized expertise in metabolic diseases to pharmaceutical and biotech partners. This fee-for-service or project-based model provides a consistent income stream, capitalizing on the company's established infrastructure and scientific knowledge.
This revenue stream is crucial for immediate financial support and allows Anaborex to leverage its core competencies. For instance, in 2024, the clinical research services sector saw significant growth, with many companies outsourcing complex trial phases. Anaborex's focus on metabolic diseases positions it well within this expanding market.
Anaborex, Inc., as an emerging biotech firm, will continue to secure vital funding through equity investments from venture capital firms and strategic investors. This is a foundational revenue stream for early-stage companies in the sector, enabling critical investments in research and development, operational expansion, and the achievement of key developmental milestones.
In 2024, the venture capital landscape for biotech remained robust, with significant capital allocated to promising early-stage companies. For instance, the Biotechnology Innovation Organization (BIO) reported that venture funding for the U.S. biotech sector reached billions of dollars in recent years, underscoring the importance of these investments for companies like Anaborex.
Grants and Non-Dilutive Funding
Anaborex, Inc. will pursue grants from government agencies like the National Institutes of Health (NIH) and the National Cancer Institute (NCI), alongside funding from disease-specific foundations. This non-dilutive funding is crucial as it supports targeted research without Anaborex having to give up ownership in the company. These grants are particularly vital for advancing Anaborex's work in areas with significant unmet medical needs, such as cancer cachexia.
Securing these grants will provide essential capital for Anaborex's research and development efforts. For instance, in 2023, the NIH awarded over $40 billion in research grants, highlighting the substantial availability of non-dilutive funding for innovative biomedical projects. Anaborex aims to tap into this by focusing on the unique therapeutic potential of its compounds for debilitating conditions.
- Government Grants: Targeting NIH and NCI funding for cancer cachexia research.
- Foundation Support: Seeking grants from organizations dedicated to cancer patient support and research.
- Non-Dilutive Capital: Acquiring funds that do not require equity dilution, preserving company ownership.
- Project-Specific Funding: Aligning grant applications with specific research milestones and therapeutic targets.
Potential Royalties from Commercialized Therapies
Anaborex, Inc. anticipates generating significant revenue through potential royalties from the commercialization of its novel therapies by partner pharmaceutical companies. These royalty payments represent a critical long-term income stream, directly tied to the market success of its developed treatments.
The financial return on Anaborex's substantial research and development investments is ultimately realized through these sales-based royalties. For instance, the global pharmaceutical market reached approximately $1.5 trillion in 2023, with innovative therapies often commanding premium pricing and substantial sales volumes.
- Royalty Payments: Anaborex will receive ongoing payments from partners based on the net sales of commercialized therapies.
- Long-Term Revenue: This stream is designed to provide sustained income well after initial development and regulatory approval.
- R&D Return: Royalties are the primary mechanism for recouping and profiting from the company's extensive R&D expenditures.
- Market Dependence: The actual revenue generated is contingent on the market adoption and sales performance of the partnered therapies.
Anaborex, Inc.'s revenue model is diversified, encompassing upfront payments, milestone achievements, and royalties from pharmaceutical licensing agreements. Additionally, the company leverages its expertise through clinical research service fees, providing a project-based income. Crucially, Anaborex also secures non-dilutive funding via government grants and foundation support, alongside essential equity investments from venture capital.
| Revenue Stream | Description | 2024 Data/Context |
| Licensing Agreements | Upfront payments, milestone payments, and royalties from pharmaceutical partners. | Milestone payments in biotech licensing can range from $50M to over $200M in 2024. |
| Clinical Research Services | Fee-for-service or project-based income for specialized metabolic disease research. | The clinical research services sector saw significant growth in 2024, with increased outsourcing of complex trials. |
| Equity Investments | Funding from venture capital firms and strategic investors. | Venture funding for the U.S. biotech sector remained robust in 2024, with billions allocated to early-stage companies. |
| Government & Foundation Grants | Non-dilutive funding for targeted research from agencies like NIH and disease-specific foundations. | NIH awarded over $40 billion in research grants in 2023, highlighting substantial non-dilutive funding availability. |
Business Model Canvas Data Sources
The Anaborex, Inc. Business Model Canvas is built upon comprehensive market research, detailed financial projections, and internal operational data. These sources ensure each block is informed by accurate, actionable insights.
