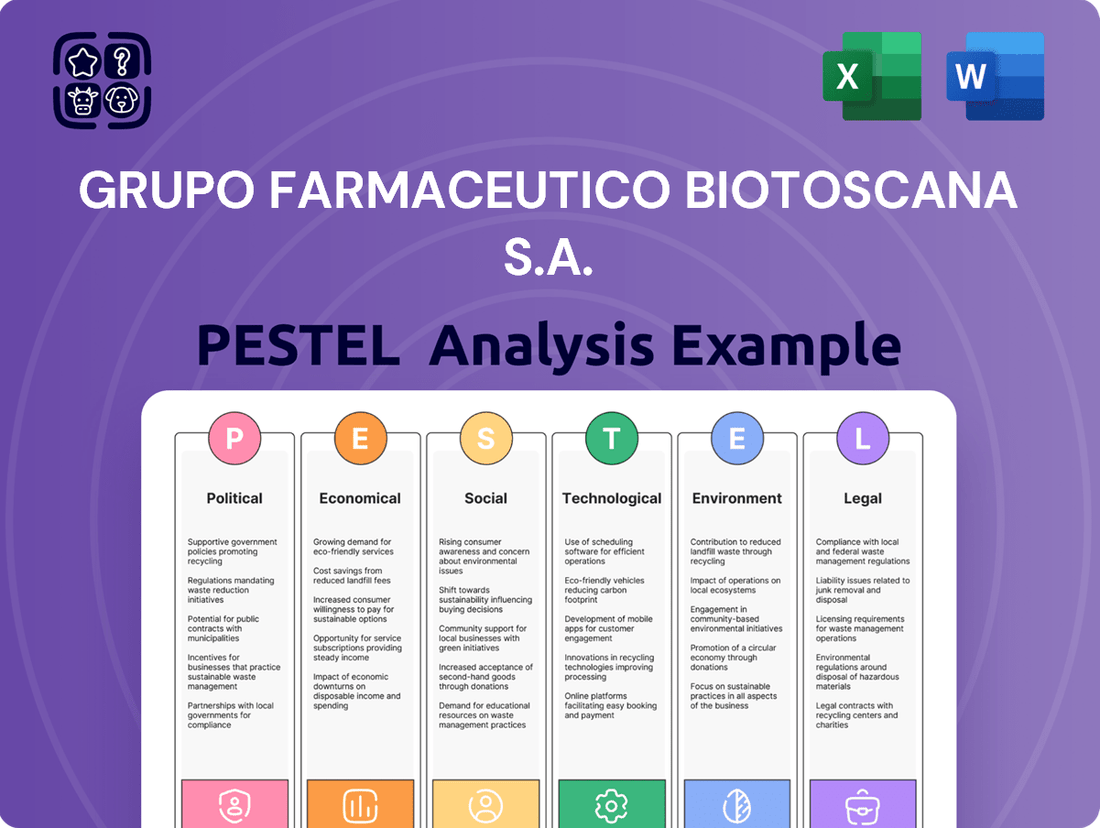
Grupo Farmaceutico Biotoscana S.A. PESTLE Analysis
Fully Editable
Tailor To Your Needs In Excel Or Sheets
Professional Design
Trusted, Industry-Standard Templates
Pre-Built
For Quick And Efficient Use
No Expertise Is Needed
Easy To Follow
Grupo Farmaceutico Biotoscana S.A. Bundle
Grupo Farmaceutico Biotoscana S.A. operates within a dynamic environment shaped by evolving political landscapes, economic fluctuations, and rapid technological advancements. Understanding these external forces is crucial for strategic planning and risk mitigation in the pharmaceutical sector. Our comprehensive PESTLE analysis delves into these critical factors, offering actionable intelligence.
Gain an edge with our in-depth PESTEL Analysis—crafted specifically for Grupo Farmaceutico Biotoscana S.A.. Discover how external forces are shaping the company’s future, and use these insights to strengthen your own market strategy. Download the full version now and get actionable intelligence at your fingertips.
Political factors
Government healthcare policies across Latin America are a major driver for Grupo Farmaceutico Biotoscana (GBT). These policies dictate everything from how drugs are bought and paid for to what health issues governments prioritize. For instance, a shift towards increased public funding for oncology treatments in Brazil, a key market for GBT, could significantly boost demand for their specialty cancer drugs.
Changes in reimbursement rates or new drug approval processes can directly affect GBT's revenue streams and how easily their products reach patients. For example, if a government decides to negotiate lower prices for imported biologics, GBT would need to adjust its market access strategies accordingly. Staying ahead of these policy shifts is vital for GBT's strategic planning and market positioning.
In 2024, many Latin American governments continued to focus on expanding access to essential medicines, with several countries reviewing their national formularies. This trend presents both opportunities and challenges for GBT, as it could increase the patient base for their innovative therapies but also intensify price pressures. For example, Colombia's health ministry announced plans in early 2024 to streamline the approval of high-cost specialty drugs, potentially benefiting GBT's portfolio.
The political stability across Latin America, where Grupo Farmaceutico Biotoscana (GBT) primarily operates, directly impacts its operational landscape. For instance, countries like Colombia and Peru, key markets for GBT, have generally maintained stable political environments in recent years, fostering a more predictable business climate. However, regional political shifts can introduce volatility, potentially affecting GBT's supply chain integrity and regulatory compliance.
Good governance and robust anti-corruption measures are crucial for fair competition within the pharmaceutical sector. In 2024, Transparency International's Corruption Perception Index highlighted varying levels of governance across Latin America. Nations with stronger rule of law and effective anti-corruption frameworks tend to offer a more level playing field for companies like GBT, mitigating risks associated with bribery or unfair market access.
Governments in Latin America, including key markets for Grupo Farmaceutico Biotoscana (GBT), wield significant influence over drug pricing and reimbursement. Policies like price caps and stringent criteria for specialty drug coverage directly affect GBT's revenue streams. For instance, in 2024, several South American nations continued to review or implement stricter price controls on pharmaceuticals, impacting the profitability of innovative treatments.
International Trade Agreements and Tariffs
Trade agreements significantly impact Grupo Farmaceutico Biotoscana (GBT) by influencing the cost and accessibility of pharmaceutical raw materials and finished goods. For instance, preferential trade agreements within Latin America can reduce import duties, making it cheaper for GBT to source components or sell its products. Conversely, new tariffs or non-tariff barriers imposed by major trading partners could increase operational costs and diminish the competitiveness of GBT's offerings in key markets.
The global trade environment is dynamic, with ongoing negotiations and potential shifts in policy. For 2024 and into 2025, GBT must closely monitor developments such as the potential expansion of the Pacific Alliance trade bloc or changes to existing agreements like Mercosur. These shifts directly affect supply chain efficiency and pricing strategies.
- Impact of Trade Agreements: Reduced tariffs on pharmaceutical imports within Latin America can lower GBT's cost of goods sold.
- Tariff and Non-Tariff Barriers: New or increased tariffs on finished products exported from GBT's manufacturing locations could raise prices for consumers and reduce demand.
- Supply Chain Optimization: Understanding trade policies is crucial for GBT to optimize sourcing and distribution networks, minimizing logistical costs and ensuring timely product availability.
- Market Access: Trade pacts can open new markets for GBT's products or create challenges in existing ones, necessitating strategic adjustments to market entry and expansion plans.
Regulatory Harmonization Efforts
Regulatory harmonization efforts across Latin America, while progressing gradually, aim to simplify drug approval pathways and lessen the operational intricacies for companies like Grupo Farmaceutico Biotoscana (GBT) when navigating diverse national markets. Any advancements in standardizing pharmaceutical regulations by regional economic blocs could significantly benefit GBT by easing compliance burdens and facilitating smoother market entry.
For instance, the Pacific Alliance, comprising Chile, Colombia, Mexico, and Peru, has been actively pursuing initiatives to align regulatory frameworks, including those pertinent to pharmaceuticals. While specific data on the direct impact of these harmonization efforts on GBT's 2024 or 2025 operational costs or approval timelines is not publicly detailed, the general trend suggests a potential for reduced administrative overhead. The World Health Organization's (WHO) efforts to promote regulatory convergence globally also play a role, indirectly influencing regional initiatives.
- Streamlined Approvals: Harmonization can reduce the number of distinct dossier requirements for each country.
- Reduced Compliance Costs: Standardized regulations lower the expense associated with meeting varying national standards.
- Faster Market Access: Simplified processes can accelerate the introduction of new GBT products to regional markets.
Government healthcare spending and policy shifts are paramount for Grupo Farmaceutico Biotoscana (GBT). In 2024, many Latin American countries continued to prioritize access to essential medicines, potentially increasing patient bases but also intensifying price pressures. For example, Colombia's early 2024 plan to expedite high-cost specialty drug approvals could benefit GBT's portfolio.
Political stability across GBT's core markets like Colombia and Peru generally supports a predictable business environment, though regional shifts can introduce volatility. Strong governance and anti-corruption measures, as highlighted by Transparency International's 2024 Corruption Perception Index, are vital for fair competition and mitigating risks.
Trade agreements significantly influence GBT's operational costs and market access. Monitoring developments in blocs like the Pacific Alliance or Mercosur is crucial for 2024-2025 supply chain optimization and pricing strategies, especially as some South American nations continued to review stricter pharmaceutical price controls in 2024.
What is included in the product
This PESTLE analysis of Grupo Farmaceutico Biotoscana S.A. examines how political stability, economic growth, social healthcare trends, technological advancements in pharmaceuticals, environmental regulations, and legal frameworks impact the company's operations and strategy.
This PESTLE analysis for Grupo Farmaceutico Biotoscana S.A. serves as a pain point reliever by offering a clear, summarized view of external factors impacting the pharmaceutical landscape, facilitating informed decision-making.
By dissecting the political, economic, social, technological, environmental, and legal influences, this analysis provides a concise framework to anticipate and mitigate potential challenges, thus easing strategic planning for Biotoscana.
Economic factors
Latin America's economic trajectory is a key driver for healthcare investment. For instance, Brazil, a major market for Grupo Farmaceutico Biotoscana (GBT), saw its GDP grow by an estimated 2.9% in 2023, with projections for 2024 indicating continued expansion. This economic vitality translates directly into increased government healthcare budgets and a greater disposable income for individuals, boosting their capacity to afford GBT's specialized treatments.
Higher economic growth in countries like Colombia and Peru, where GBT also operates, directly fuels healthcare spending. In 2024, many Latin American economies are expected to maintain positive growth trends, creating a more favorable environment for pharmaceutical companies. This increased spending capacity enhances demand for GBT's high-value oncology and hematology products, as both public health systems and private payers can allocate more resources to advanced medical care.
High inflation rates and volatile currency exchange rates in Latin America present considerable economic hurdles for Grupo Farmaceutico Biotoscana (GBT). For instance, Argentina experienced an average inflation rate of over 200% in 2023, and projections for 2024 remain elevated, significantly impacting GBT's operational costs.
Currency fluctuations further complicate matters. The Argentine peso depreciated by approximately 50% against the US dollar in 2023 alone. This volatility directly affects the cost of imported raw materials and the reported value of GBT's earnings when translated into its reporting currency, demanding robust risk management strategies to maintain financial stability.
The level of investment in healthcare infrastructure, such as hospitals and clinics, directly influences how easily Grupo Farmaceutico Biotoscana (GBT) therapies can reach patients. In 2024, global healthcare infrastructure spending is projected to reach $1.5 trillion, with significant growth expected in emerging markets.
Countries that are actively enhancing their healthcare systems, including building more specialized treatment centers, create a more favorable environment for GBT's complex treatments. This expansion of accessibility broadens the potential market for the company's innovative medicines.
Both public sector funding and private sector investment are crucial in driving these infrastructure improvements. For instance, government initiatives in Latin America, GBT's primary market, are increasingly prioritizing healthcare modernization, with public health expenditure rising by an average of 5% annually in key countries.
Patient Purchasing Power and Affordability
The economic capacity of patients to acquire GBT's specialty medications, even with insurance, is a critical factor. This affordability is directly tied to income levels, the comprehensiveness of health insurance plans, and the patient's personal financial contribution. For instance, in 2024, the average out-of-pocket cost for specialty drugs in some Latin American markets could represent a significant portion of household income, impacting access for a substantial number of individuals.
Understanding these economic realities is paramount for GBT's market strategy. Factors influencing patient purchasing power include:
- Median Household Income: Variations in income across target countries directly affect how much patients can spend on healthcare.
- Insurance Penetration Rates: The percentage of the population with robust health insurance coverage influences the number of patients who can afford GBT's products.
- Co-payment and Deductible Structures: The specific terms of insurance plans, particularly out-of-pocket expenses, can be a major barrier to treatment adherence.
Competition and Market Dynamics
The competitive intensity within the Latin American pharmaceutical market significantly impacts Grupo Farmaceutico Biotoscana S.A. (GBT). The presence of numerous generic manufacturers, alongside established biopharmaceutical companies and the continuous entry of new players, exerts considerable pressure on pricing strategies and market share for GBT.
Market dynamics, including consolidation and fragmentation, further shape the competitive environment. For instance, in 2024, the Latin American pharmaceutical market experienced a notable trend towards consolidation, with several mid-sized companies being acquired, potentially altering the competitive landscape for GBT.
- Increased Competition: The Latin American pharmaceutical market is characterized by a high number of local and international players, intensifying price competition.
- Generic Drug Penetration: The growing market share of generic drugs in key LATAM markets, such as Brazil and Mexico, puts pressure on branded pharmaceutical sales for companies like GBT.
- Market Consolidation Trends: In 2024, M&A activity in the LATAM pharma sector saw increased interest, with companies looking to expand their portfolios and market reach, potentially leading to fewer, larger competitors.
- New Entrants: Emerging biotech firms and specialized pharmaceutical companies continue to enter the region, introducing innovative products and challenging established market positions.
Grupo Farmaceutico Biotoscana (GBT) operates within a dynamic economic landscape in Latin America, where GDP growth directly correlates with healthcare spending. For example, Brazil's projected GDP growth of around 2.5% for 2024 fuels increased public health budgets and individual purchasing power for GBT's specialized treatments.
However, high inflation and currency volatility, particularly in countries like Argentina which saw inflation exceed 200% in 2023, pose significant challenges to GBT's operational costs and the valuation of its earnings. Currency depreciation, such as the Argentine peso's roughly 50% drop against the USD in 2023, directly impacts the cost of imported materials.
Healthcare infrastructure investment, projected to reach $1.5 trillion globally in 2024 with notable growth in emerging markets, is crucial for GBT. Enhanced healthcare systems in Latin America, supported by an average 5% annual rise in public health expenditure in key nations, improve access to GBT's advanced therapies.
Patient affordability, tied to median household income, insurance penetration, and co-payment structures, remains a critical factor. In 2024, the out-of-pocket cost for specialty drugs in some LATAM markets can be substantial, impacting treatment adherence.
| Country | Estimated 2024 GDP Growth (%) | 2023 Avg. Inflation (%) | Currency Depreciation vs. USD (2023) |
|---|---|---|---|
| Brazil | 2.5 | 4.6 | -1.5 |
| Argentina | 2.0 | 211.4 | -50.0 |
| Colombia | 3.0 | 9.3 | -15.0 |
Preview Before You Purchase
Grupo Farmaceutico Biotoscana S.A. PESTLE Analysis
The preview shown here is the exact document you’ll receive after purchase—fully formatted and ready to use, detailing the PESTLE analysis for Grupo Farmaceutico Biotoscana S.A. This comprehensive report covers Political, Economic, Social, Technological, Legal, and Environmental factors impacting the company.
Sociological factors
The increasing prevalence of chronic diseases like cancer and hematological disorders across Latin America is a significant driver for Grupo Farmaceutico Biotoscana (GBT). For instance, in 2023, the World Health Organization reported that cancer was the second leading cause of death in the region, with over 1.4 million new cases diagnosed annually, directly boosting demand for GBT's oncology and hematology treatments.
Demographic shifts, particularly the aging population in key Latin American markets, further amplify this demand. By 2025, it's projected that over 15% of the population in countries like Brazil and Argentina will be aged 65 and above, a demographic group historically more susceptible to the types of conditions GBT specializes in treating. This trend expands GBT's potential patient base and market opportunities.
Patient awareness significantly shapes healthcare demand. In 2024, a significant portion of the Latin American population, particularly in countries where GBT operates, showed increased interest in understanding chronic disease management and new treatment pathways. This growing health literacy, fueled by digital health platforms and accessible medical information, directly impacts the uptake of specialized pharmaceutical products.
Public health education campaigns in 2025 are expected to further elevate patient engagement with preventative care and early diagnosis. For Grupo Farmaceutico Biotoscana S.A., this translates to a potential surge in demand for their innovative therapies, as more patients become aware of and seek out advanced treatment options for conditions like cancer and autoimmune diseases. This trend is a positive indicator for GBT's market penetration and revenue growth.
Sociological factors significantly influence Grupo Farmaceutico Biotoscana S.A. (GBT), particularly concerning access to healthcare. The availability of specialized oncology and hematology centers, crucial for GBT's product portfolio, varies greatly. For instance, while major urban centers in Latin America often boast advanced medical facilities, rural and remote areas frequently face shortages of both equipment and highly trained personnel, impacting the reach of GBT's innovative treatments.
Disparities in access are a persistent challenge. In 2024, reports indicated that a substantial portion of the population in certain Latin American countries still lacked consistent access to specialized medical care, a direct consequence of geographic isolation and economic inequality. This uneven distribution of healthcare resources directly affects GBT's market penetration and patient support initiatives.
GBT's strategic focus on patient access programs directly addresses these sociological hurdles. By investing in initiatives aimed at improving healthcare infrastructure and training medical professionals, GBT seeks to bridge the gap between urban and rural populations, ensuring more equitable distribution of its life-saving medicines. This commitment is vital for fostering trust and expanding its positive impact.
Cultural Perceptions of Health and Medicine
Cultural perceptions significantly shape how patients in Latin America view modern medicine versus traditional or alternative treatments, directly influencing their willingness to adopt Grupo Farmaceutico Biotoscana's (GBT) innovative therapies. For instance, in some regions, there's a deep-seated trust in natural remedies, which can lead to slower adoption rates for pharmaceuticals, even those with strong clinical backing. This cultural inclination means GBT must tailor its patient education and marketing strategies to bridge this gap, highlighting the scientific efficacy and safety of its products in a culturally sensitive manner.
Patient adherence to GBT's treatment regimens is also heavily influenced by cultural attitudes towards health and illness. In 2024, surveys across several Latin American countries indicated that while awareness of advanced medical treatments is growing, a substantial portion of the population still prioritizes community-based health practices or relies on advice from local healers before consulting formal medical channels. This underscores the need for GBT to build trust not only in its products but also in the broader healthcare systems that deliver them, often through partnerships with local healthcare providers and community leaders.
The adoption of new medical technologies and therapies by patients is often a reflection of their overall trust in the healthcare system and the pharmaceutical industry. In 2025, reports suggest that while patient satisfaction with healthcare services is improving in key GBT markets, concerns about affordability and accessibility persist, often exacerbated by cultural beliefs about who should provide care. Therefore, GBT's success hinges on its ability to demonstrate not just product value but also a commitment to equitable access and transparent communication, aligning with cultural expectations of care and support.
Key cultural considerations impacting GBT's market penetration include:
- Varying levels of trust in Western medicine versus traditional healing practices across different Latin American demographics.
- The influence of family and community elders on healthcare decisions, often prioritizing collective well-being over individual medical advice.
- Cultural narratives surrounding specific diseases and their treatments, which can foster or hinder the acceptance of pharmaceutical interventions.
- Patient expectations regarding the doctor-patient relationship, with some cultures valuing a more paternalistic approach while others prefer shared decision-making.
Socioeconomic Disparities and Health Equity
Socioeconomic disparities across Latin America significantly impact health equity, directly influencing who can access and afford Grupo Farmaceutico Biotoscana S.A.'s (GBT) specialized and often high-value medicines. For instance, in 2024, the World Bank highlighted that over 60 million people in Latin America and the Caribbean still live below the poverty line, a demographic that struggles with even basic healthcare expenses, let alone advanced therapies.
GBT's commitment to enhancing patient access necessitates a strategic approach to these ingrained disparities. The company may need to implement or expand patient assistance programs, offer tiered pricing models, or forge partnerships with governments and NGOs to ensure its innovative treatments reach a wider patient base. By doing so, GBT can actively contribute to bridging the gap in health outcomes.
- Health Inequity: Socioeconomic gaps in Latin America mean that access to advanced pharmaceuticals like GBT's is often limited to higher-income segments of the population.
- Access Challenges: In 2024, an estimated 30% of the Latin American population faced financial barriers to accessing essential medicines, a figure that likely rises for specialized treatments.
- GBT's Role: GBT's mission to improve patient access requires navigating these socioeconomic hurdles, potentially through patient support initiatives and collaborations for wider therapy availability.
- Sociological Imperative: Addressing health equity is a critical sociological consideration for pharmaceutical companies operating in regions with significant income inequality.
Societal attitudes towards health and wellness in Latin America significantly shape the market for Grupo Farmaceutico Biotoscana S.A. (GBT). For example, a growing emphasis on preventative care and healthy lifestyles, observed across many of GBT's key markets in 2024, could influence demand for certain therapeutic areas while potentially impacting others. This evolving health consciousness means GBT must remain agile in aligning its product portfolio and marketing efforts with prevailing societal values and health priorities.
Furthermore, the influence of social media and digital health platforms is increasingly shaping patient perceptions and treatment choices. By 2025, it's projected that a substantial portion of the population will rely on online resources for health information, creating opportunities for GBT to engage directly with patients and healthcare providers through digital channels. This digital engagement is crucial for disseminating information about GBT's specialized treatments and fostering informed decision-making.
The role of patient advocacy groups is also a critical sociological factor. These groups, active in raising awareness and lobbying for better access to treatments for conditions like cancer and rare diseases, can exert considerable influence on public policy and market dynamics. GBT's engagement with these organizations, as seen in ongoing collaborations in 2024, is vital for understanding patient needs and advocating for supportive regulatory environments.
Key sociological trends impacting GBT:
| Trend | Description | Impact on GBT |
|---|---|---|
| Preventative Health Emphasis | Growing societal focus on proactive health management and wellness. | May shift demand towards treatments for lifestyle-related conditions or require GBT to highlight the long-term benefits of its therapies. |
| Digital Health Adoption | Increased reliance on online platforms for health information and consultation. | Creates opportunities for targeted digital marketing and patient education campaigns. |
| Patient Advocacy Influence | Growing power of patient groups in shaping healthcare policy and access. | Requires GBT to actively engage with advocacy groups to understand patient needs and support access initiatives. |
Technological factors
Rapid advancements in biopharmaceutical R&D, such as gene therapies and immunotherapies, are reshaping the competitive landscape. GBT's ability to integrate these innovations into its oncology and hematology portfolios is crucial for maintaining its edge. For instance, the global biopharmaceutical R&D spending reached an estimated $210 billion in 2024, highlighting the intense innovation cycle.
Staying ahead requires significant investment in R&D. GBT's focus on developing cutting-edge therapies means that continued investment in areas like targeted drug delivery systems is paramount to its future success and market positioning.
Grupo Farmaceutico Biotoscana (GBT) can significantly boost its drug production efficiency and cost-effectiveness through the adoption of advanced manufacturing technologies. Embracing continuous manufacturing, automation, and refined bioprocessing techniques are key to this enhancement. These innovations are projected to streamline production cycles, leading to a more competitive cost structure for GBT's pharmaceutical products.
The growing adoption of digital health and telemedicine is a significant technological factor for Grupo Farmaceutico Biotoscana (GBT). These solutions, including remote patient monitoring and AI diagnostics, are enhancing patient care and broadening access to medical expertise. For instance, by mid-2024, telemedicine consultations in Latin America were projected to see a substantial increase, with some markets experiencing growth rates exceeding 30% year-over-year, as reported by various regional health tech analyses.
GBT can capitalize on these advancements to bolster patient engagement with its treatments. By integrating digital platforms, the company can deliver educational materials and monitor patient adherence more effectively. This is particularly relevant for GBT's operations across Latin America, where expanding the reach of its therapies to remote or underserved populations can be significantly aided by these digital tools, potentially improving treatment outcomes and market penetration.
Data Analytics and Personalized Medicine
The pharmaceutical industry is increasingly leveraging data analytics and artificial intelligence to accelerate drug discovery and development. GBT can harness these advanced tools to pinpoint patient subgroups most likely to respond to its treatments, thereby enhancing therapeutic efficacy and enabling personalized medicine approaches. This data-driven strategy also strengthens the generation of real-world evidence, crucial for market access and demonstrating value.
The integration of AI in clinical trials is proving transformative. For instance, by mid-2024, many leading biopharmaceutical companies reported significant reductions in trial timelines, with some AI-driven patient recruitment platforms showing up to a 30% improvement in identifying eligible participants. GBT can adopt similar technologies to optimize its clinical trial design and execution, potentially reducing costs and speeding up the path to market for its innovative therapies.
- AI in Drug Discovery: Companies are seeing up to a 25% increase in the identification of novel drug candidates through AI-powered screening by early 2025.
- Personalized Medicine Adoption: The market for personalized medicine is projected to reach over $700 billion globally by 2028, indicating substantial growth potential.
- Real-World Evidence (RWE): RWE studies are becoming critical for regulatory submissions, with a reported 40% increase in their use in pharmaceutical decision-making between 2022 and 2024.
Supply Chain Digitization and Traceability
Technological advancements are significantly reshaping pharmaceutical supply chains, offering enhanced security and efficiency. For Grupo Farmaceutico Biotoscana S.A. (GBT), embracing these changes is crucial, especially given the complexities of the Latin American market.
Blockchain technology, for instance, offers unparalleled traceability for pharmaceuticals, a critical factor in combating counterfeit drugs. This not only ensures product integrity but also builds consumer trust. Advanced logistics software further optimizes inventory management, reducing waste and improving delivery times.
The global pharmaceutical supply chain market is projected to grow substantially, with digital solutions playing a key role. For example, the market for pharmaceutical supply chain management software was valued at approximately $1.5 billion in 2023 and is expected to reach over $3.5 billion by 2030, indicating a strong trend towards digitization.
- Blockchain for Drug Traceability: Enhances security and transparency, helping to combat the estimated 10% of medicines in low- and middle-income countries being substandard or falsified, a figure reported by the WHO.
- Advanced Logistics Software: Optimizes inventory and distribution, crucial for GBT's operations across diverse Latin American geographies.
- Data Analytics in Supply Chains: Enables better demand forecasting and risk management, leading to more efficient operations.
- IoT in Warehousing: Improves real-time monitoring of storage conditions, ensuring product quality and compliance.
Technological advancements in drug discovery, particularly AI, are accelerating the identification of new therapeutic candidates. By early 2025, companies are seeing up to a 25% increase in novel drug discoveries through AI-powered screening. GBT can leverage these tools to enhance its R&D pipeline, focusing on areas like oncology and hematology.
The rise of digital health and telemedicine is transforming patient care and access, with telemedicine consultations in Latin America projected to grow over 30% year-over-year by mid-2024. GBT can integrate these platforms to improve patient engagement and treatment adherence, especially in remote regions.
AI's impact on clinical trials is significant, with AI-driven patient recruitment platforms showing up to a 30% improvement in identifying eligible participants by mid-2024. Adopting similar technologies can streamline GBT's trial processes, potentially reducing costs and accelerating market entry.
Supply chain digitization, including blockchain for traceability, is crucial for combating counterfeit drugs, which affect an estimated 10% of medicines in low- and middle-income countries according to the WHO. GBT's adoption of advanced logistics and data analytics will enhance efficiency and product integrity.
| Technology Area | Impact on GBT | 2024/2025 Data Point |
|---|---|---|
| AI in Drug Discovery | Accelerated candidate identification | Up to 25% increase in novel drug candidates identified by early 2025. |
| Digital Health/Telemedicine | Improved patient engagement and access | Over 30% year-over-year growth in Latin American telemedicine consultations projected by mid-2024. |
| AI in Clinical Trials | Faster trial execution | Up to 30% improvement in participant identification via AI platforms by mid-2024. |
| Blockchain for Traceability | Enhanced product security and trust | Combats counterfeit drugs, affecting ~10% of medicines in LMICs (WHO). |
Legal factors
The drug approval and registration processes across Latin America present a significant legal challenge for Grupo Farmaceutico Biotoscana (GBT). Each country has its own set of requirements, demanding extensive documentation and rigorous clinical data to bring new therapies to market. For instance, in 2024, the time to register a new drug in Brazil, a key market for GBT, averaged 480 days, while in Argentina, it could extend to over 600 days, highlighting the operational complexities.
Compliance with local health authorities, such as ANVISA in Brazil and COFEPRIS in Mexico, is absolutely critical. GBT must meticulously adhere to these varying regulations, which often involve lengthy review periods and specific bioequivalence studies. Failure to navigate these intricate legal pathways can lead to significant delays and increased costs, impacting GBT's ability to compete effectively in these vital markets.
Grupo Farmaceutico Biotoscana S.A. (GBT) relies heavily on robust intellectual property rights, particularly patents for its novel drug formulations and production methods. These patents are the bedrock of its business, allowing it to recoup significant research and development expenditures.
The legal landscape for patent enforcement and data exclusivity across Latin America is crucial for GBT. In 2024, for instance, countries like Brazil and Colombia continued to refine their patent laws, impacting how long GBT can maintain market exclusivity for its specialized pharmaceuticals, thereby influencing its competitive advantage and pricing power.
Grupo Farmaceutico Biotoscana S.A. (GBT) operates under a stringent legal framework for pharmaceutical advertising and marketing across Latin America, especially concerning prescription and specialty drugs. Compliance with local regulations on claims, endorsements, and direct-to-consumer advertising is paramount to avoid severe penalties.
Failure to adhere to these advertising laws can result in substantial fines and significant damage to GBT's reputation. For instance, in 2024, several Latin American countries increased enforcement actions against pharmaceutical companies for misleading marketing claims, with fines often reaching millions of dollars.
Anti-Corruption and Compliance Laws
Grupo Farmaceutico Biotoscana S.A. (GBT) must navigate a complex legal landscape, particularly concerning anti-corruption and compliance. Operating across Latin America means strict adherence to regulations like the U.S. Foreign Corrupt Practices Act (FCPA) and numerous local anti-bribery statutes is paramount. Failure to comply can lead to severe penalties.
To mitigate these risks, GBT is compelled to establish and maintain comprehensive compliance programs. These programs are designed to prevent unethical practices, including bribery, in all dealings with healthcare professionals, government officials, and other stakeholders. The legal ramifications of corruption are substantial, impacting reputation and financial stability.
- FCPA Enforcement: In 2023, the U.S. Department of Justice reported significant enforcement actions under the FCPA, highlighting the ongoing scrutiny on companies operating internationally.
- Compliance Investment: Companies in the pharmaceutical sector globally are increasing their investment in compliance training and monitoring, with many allocating over 5% of their annual compliance budget to anti-corruption initiatives.
- Local Regulations: GBT must also comply with specific anti-corruption laws in each country of operation, such as Brazil's Law No. 12.846/2013 (Clean Company Act), which imposes strict liability on companies for corrupt acts.
Product Liability and Consumer Protection Laws
Product liability laws are a significant consideration for Grupo Farmaceutico Biotoscana S.A. (GBT), as they hold pharmaceutical firms accountable for ensuring the safety and effectiveness of their medications. This means GBT must rigorously adhere to regulations designed to protect consumers, which include mandates for transparent labeling, prompt reporting of any adverse events, and well-defined procedures for product recalls. For instance, in 2024, the pharmaceutical industry globally saw significant settlements and fines related to product safety issues, highlighting the potential financial ramifications. Failing to meet these stringent legal requirements can lead to substantial financial penalties and severe damage to the company's reputation, impacting market trust and future sales.
Consumer protection regulations further shape GBT's operational landscape. These laws are in place to guarantee that consumers receive accurate information about the products they purchase and use. For GBT, this translates to a need for clear, unambiguous product labeling that details ingredients, dosage, potential side effects, and contraindications. Furthermore, robust systems for monitoring and reporting adverse drug reactions are critical, as is the capacity to execute swift and effective product recalls if safety concerns arise. Reports from the World Health Organization in 2024 emphasized the increasing focus on pharmacovigilance across emerging markets, where GBT has a significant presence.
The legal challenges stemming from product safety issues can indeed carry immense weight for pharmaceutical companies. These can range from individual lawsuits filed by consumers alleging harm from a product to class-action suits and governmental regulatory actions. The financial consequences can be staggering, encompassing damages awarded to plaintiffs, legal defense costs, and regulatory fines. Beyond the direct financial impact, the reputational damage from a product liability scandal can erode consumer confidence, deter investment, and negatively affect relationships with healthcare providers and distributors. For example, in late 2024, a major pharmaceutical recall resulted in an estimated $500 million in costs for the involved company, including lost sales and remediation efforts.
- Product Safety Mandates: GBT must comply with laws requiring rigorous testing and validation to prove the safety and efficacy of its pharmaceutical products before they reach the market.
- Consumer Transparency: Regulations necessitate clear and accurate product labeling, including detailed information on usage, potential side effects, and contraindications, to empower informed consumer choices.
- Adverse Event Reporting: GBT is legally obligated to establish and maintain systems for timely and accurate reporting of any adverse events experienced by patients using its medications.
- Recall Preparedness: The company must have established procedures for efficiently and effectively recalling products from the market should safety concerns or defects be identified.
Grupo Farmaceutico Biotoscana S.A. (GBT) navigates a complex web of legal and regulatory frameworks across Latin America, impacting everything from drug approval to marketing practices. Strict adherence to varying country-specific health authority guidelines, such as ANVISA in Brazil, is essential for market access, with registration processes in 2024 averaging 480 days in Brazil and exceeding 600 days in Argentina.
Intellectual property rights, particularly patents, are critical for GBT to protect its innovations and recoup R&D investments. The legal landscape for patent enforcement and data exclusivity in 2024 continued to evolve in key markets like Brazil and Colombia, directly influencing GBT's competitive edge and pricing strategies.
Furthermore, GBT faces stringent regulations on pharmaceutical advertising and marketing, with increased enforcement actions observed in 2024 for misleading claims, often resulting in substantial fines. Compliance with anti-corruption laws, including the FCPA and local statutes like Brazil's Clean Company Act, is paramount, with companies globally increasing compliance budgets to over 5% of their annual compliance spend on anti-corruption initiatives in 2023.
Environmental factors
Grupo Farmaceutico Biotoscana S.A. (GBT) faces significant environmental challenges due to the nature of pharmaceutical manufacturing, which inherently produces chemical and biological waste. Compliance with diverse national and international regulations for hazardous waste disposal is paramount, impacting operational costs and strategic planning.
In 2024, the global pharmaceutical waste management market was valued at approximately USD 12.5 billion, with projections indicating continued growth driven by stricter environmental laws. GBT must navigate these evolving regulations across its operating regions, ensuring safe and compliant disposal methods for all generated by-products.
Adopting sustainable waste management practices is no longer just a regulatory necessity but a key component of corporate social responsibility. Companies like GBT are increasingly investing in waste reduction technologies and circular economy principles to minimize their environmental footprint and enhance their reputation, potentially improving investor relations and market access.
Grupo Farmaceutico Biotoscana S.A. (GBT) faces significant environmental considerations, particularly concerning its energy consumption and resulting carbon footprint. The energy demands of its manufacturing plants, extensive research and development facilities, and complex logistics networks directly contribute to its overall environmental impact.
Growing global pressure for environmental stewardship, coupled with the increasing likelihood of carbon taxes, compels GBT to prioritize energy efficiency. This includes investing in more sustainable operational practices and actively exploring renewable energy sources to mitigate its environmental impact and potentially lower operational costs. For instance, many pharmaceutical companies are setting ambitious targets; by 2025, several leading firms aim to source 50% or more of their electricity from renewables.
Furthermore, robust sustainability reporting is rapidly evolving from a voluntary initiative to an industry standard. GBT's transparency in disclosing its energy consumption and carbon emissions, along with its strategies for reduction, will be crucial for maintaining stakeholder trust and complying with emerging regulatory frameworks.
Pharmaceutical manufacturing, including for companies like Grupo Farmaceutico Biotoscana S.A. (GBT), is typically a significant consumer of water. This high water demand is driven by various processes such as cleaning, cooling, and as a component in formulations. For instance, the global pharmaceutical industry's water footprint is substantial, with some estimates suggesting that producing a kilogram of active pharmaceutical ingredient (API) can require thousands of liters of water.
The discharge of wastewater from pharmaceutical facilities is a critical environmental concern, particularly when it contains residual active pharmaceutical ingredients (APIs). These compounds, even in trace amounts, can pose risks to aquatic ecosystems and potentially human health if not properly treated. GBT, like other players in the sector, faces increasing scrutiny and regulatory pressure to manage these discharges effectively.
To address these challenges, GBT must not only comply with stringent water usage regulations but also proactively invest in advanced wastewater treatment technologies. Such investments are crucial for minimizing the company's ecological footprint and ensuring adherence to evolving environmental standards, which are becoming more rigorous worldwide. For example, many regions are implementing stricter limits on API concentrations in discharged water, pushing companies towards more sophisticated filtration and degradation methods.
Supply Chain Environmental Impact
Grupo Farmaceutico Biotoscana S.A. (GBT) must acknowledge that its environmental impact stretches far beyond its own facilities, encompassing the entire supply chain. This includes the environmental toll of sourcing raw materials, the carbon emissions from transporting goods, and the waste generated by packaging. As of 2024, companies across all sectors are facing heightened scrutiny regarding their extended environmental responsibilities.
GBT is under increasing pressure from regulators, investors, and consumers to actively assess and reduce the environmental footprint of its suppliers and its complex distribution networks. This trend is particularly pronounced in the pharmaceutical industry, where the lifecycle impact of products is under a microscope. For instance, a 2024 report by the European Environment Agency highlighted that logistics and supply chain activities account for a significant portion of greenhouse gas emissions in manufacturing sectors.
- Sustainable Sourcing: GBT needs to implement robust programs to ensure its raw material suppliers adhere to environmental standards, potentially reducing water usage and chemical runoff.
- Green Logistics: Exploring options like optimizing shipping routes, utilizing more fuel-efficient transport, and potentially shifting towards lower-emission vehicles for distribution are crucial.
- Packaging Innovation: Reducing single-use plastics and exploring biodegradable or recyclable packaging materials for its pharmaceutical products will be a key area of focus.
- Supplier Audits: Establishing regular environmental performance audits for key suppliers will be essential to drive accountability and improvement throughout the value chain.
Climate Change Adaptation and Resilience
Climate change presents significant risks to Grupo Farmaceutico Biotoscana (GBT) operations. Extreme weather events, such as floods or droughts, could disrupt critical supply chains, impacting the availability of raw materials and the distribution of finished pharmaceutical products. For instance, a severe heatwave in a key agricultural region could affect the sourcing of botanical ingredients, a potential concern for some biopharmaceutical companies.
To mitigate these risks, GBT must prioritize climate change adaptation and build resilience across its operations and supply chain. This involves developing contingency plans for manufacturing and distribution to ensure continuity even when faced with environmental disruptions. A proactive approach could involve diversifying suppliers or investing in climate-resilient infrastructure.
Furthermore, GBT needs to carefully assess the evolving landscape of climate policies. New regulations related to carbon emissions, water usage, or waste management could impact operational costs and compliance requirements. For example, stricter regulations on pharmaceutical waste disposal, driven by climate concerns, could necessitate investments in new treatment technologies.
- Supply Chain Vulnerability: Extreme weather events in 2024, such as widespread droughts in South America, impacted agricultural yields, potentially affecting raw material sourcing for pharmaceutical ingredients.
- Resilience Strategies: Companies like GBT are increasingly exploring diversified sourcing and investing in climate-resilient logistics to counter supply chain disruptions.
- Policy Impact: Anticipated carbon pricing mechanisms or enhanced environmental reporting mandates, expected to be implemented or strengthened in various Latin American markets by 2025, could influence GBT's operational expenditures.
Grupo Farmaceutico Biotoscana S.A. (GBT) faces significant environmental challenges, including managing pharmaceutical waste and its carbon footprint. The company must comply with stringent regulations regarding hazardous waste disposal and increasing pressure for sustainable energy practices. For instance, the global pharmaceutical waste management market is projected to grow significantly, underscoring the importance of efficient waste handling.
Water usage and wastewater discharge are critical concerns, especially with residual active pharmaceutical ingredients (APIs) in effluent. GBT needs to invest in advanced wastewater treatment to meet evolving environmental standards and minimize ecological impact. The industry's substantial water footprint highlights the need for responsible water management strategies.
The company's environmental impact extends to its supply chain, necessitating sustainable sourcing, green logistics, and packaging innovation. As of 2024, supply chain environmental scrutiny is intensifying, with reports indicating significant greenhouse gas emissions from logistics activities across manufacturing sectors.
Climate change poses risks to GBT's operations through extreme weather events that can disrupt supply chains. Adaptation strategies, such as diversifying suppliers and investing in resilient infrastructure, are crucial. Anticipated climate policies, including potential carbon pricing, could also affect operational costs by 2025.
PESTLE Analysis Data Sources
Our PESTLE analysis for Grupo Farmaceutico Biotoscana S.A. is informed by a comprehensive review of official regulatory filings, pharmaceutical industry association reports, and reputable financial news outlets. This ensures our insights into political, economic, social, technological, legal, and environmental factors are grounded in verifiable information.
